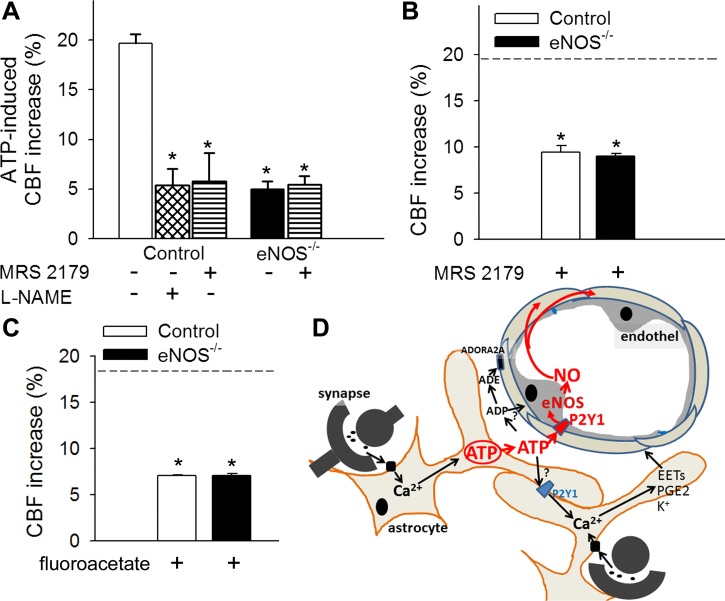
Fig. 3.
ATP-mediated CBF responses, role of P2Y1 receptors in NO mediation of neurovascular coupling. A: increases in CBF (expressed as a percentage of baseline) in the barrel field of the primary somatosensory cortex of control and eNOS−/− mice in response to topical administration of ATP in the presence and absence of the potent, selective, competitive P2Y1 receptor antagonist MRS2179 (6 × 10−5 mol/l for 20 min) or the NOS inhibitor l-NAME (10−4 mol/l for 20 min) (n = 6, *P < 0.05 vs. control). CBF was measured with a laser Doppler probe above the whisker barrel cortex. B and C: increases in CBF in the barrel field of the primary somatosensory cortex of control and eNOS−/− mice induced by contralateral whisker stimulation (5 Hz) in the presence of MRS2179 (6 × 10−5 mol/l for 20 min; B) or the glial-specific metabolic toxin fluoroacetate (3 × 10−4 mol/l for 20 min, C). Dashed line indicates the mean of the control responses (n = 6, *P < 0.05 vs. control). Data are means ± SE. D: scheme depicting purinergic glio-endothelial coupling during neuronal activity, highlighting the role of ATP-mediated activation of eNOS via P2Y1 receptors in functional hyperemia. The model acknowledges that multiple parallel astrocytic pathways exist [including synthesis of epoxyeicosatrienoic acids (EETs), prostaglandins (PGE2), and release of K+, which hyperpolarizes vascular smooth muscle cells] that are activated simultaneously to regulate blood flow and that ATP and its hydrolysis products [ADP and adenosine (ADE)] can evoke arteriolar dilation via adenosine A2 receptors located on the smooth muscle cells, as well.