Abstract
Obesity in pregnancy is associated with increased fetal growth and adiposity, which, in part, is determined by transplacental nutrient supply. Trophoblast uptake and intracellular trafficking of lipids are dependent on placental fatty acid transport proteins (FATP), translocase (FAT/CD36), and fatty acid binding proteins (FABP). We hypothesized that maternal obesity in mice leads to increased placental expression of FAT/CD36, FATPs, and FABPs, and lipid accumulation in the fetal liver. C57/BL6J female mice were fed either a control (C; n = 10) or an obesogenic (OB; n = 10) high-fat, high-sugar diet before mating and throughout pregnancy. At E18.5, placentas and fetal livers were collected. Trophoblast plasma membranes (TPM) were isolated from placental homogenates. Expression of FAT/CD36 and FATP (TPM) and FABP (homogenates) was determined by immunoblotting. Gene expression was assessed by RT-quantitative PCR. Sections of fetal livers were stained for Oil Red O, and lipid droplets were quantified. TPM protein expression of FAT/CD36, FATP 2, and FATP 4 was comparable between C and OB groups. Conversely, TPM FATP 6 expression was increased by 35% in OB compared with C placentas without changes in mRNA expression. FABPs 1, 3–5 and PPARγ were expressed in homogenates, and FABP 3 expression increased 27% in OB compared with C placentas; however, no changes were observed in mRNA expression. Lipid droplet accumulation was 10-fold higher in the livers of fetuses from OB compared with C group. We propose that increased lipid transport capacity in obese mice promotes transplacental fatty acid transport and contributes to excess lipid accumulation in the fetal liver.
Keywords: trophoblast, fatty acid, maternal-fetal exchange, fatty acid transport protein
maternal obesity increases the risk for delivering a large baby (37), neonatal adiposity (12, 55), and intrahepatic lipid accumulation (7, 42), which are associated with development of metabolic syndrome in the adult offspring (3, 45). Indeed, the increasing incidence of nonalcoholic fatty liver disease (NAFLD) in neonates (7) could contribute to the progression of metabolic disease in adult life (59). The strong association between maternal obesity in pregnancy and development of metabolic syndrome in adulthood is of particular concern because almost two-thirds of pregnant women in the United States are either overweight or obese (44).
Fetal growth is dependent on fetal nutrient availability, which is largely determined by the capacity of the placenta to transport nutrients. Placental transport of fatty acids is critical for fetal growth, particularly in late gestation, when deposition of fat in the fetus increases exponentially (36). Maternal circulating concentrations of triacylglycerides (TAG) increase 2.5-fold across gestation (13), and maternal TAGs are a major substrate for placental lipases localized to the syncytiotrophoblast microvillous membrane (MVM), which releases free fatty acids for subsequent transport to the fetus (17, 20, 31). Fetal tissue deposition of long-chain polyunsaturated fatty acids depends on the quantity and composition of fatty acids delivered via the placenta. However, the mechanisms that determine transplacental lipid transfer to the fetus are not well established.
The exact mechanisms by which fatty acids are transferred across the syncytiotrophoblast plasma membranes (MVM and basal plasma membrane, BM) are poorly understood. However, a glycosylated fatty acid translocase (FAT/CD36) (1), which is expressed in primary trophoblasts and in isolated MVM and BM (9, 17), and members of the solute carrier family 27 (SLC27A1-6) known as fatty acid transport proteins (FATP 1–6) (21, 34, 35) have been proposed to mediate transplacental transport of fatty acids. FATP 1 and FAT/CD36 proteins were first identified in syncytiotrophoblast membranes (9). Subsequently, other FATP isoforms were detected in human placental homogenates and primary trophoblasts at the mRNA (34, 35, 41, 52) and protein level (16).
Free fatty acids taken up into the syncytiotrophoblast cytoplasm are bound to fatty acid binding proteins (FABP) (9). Members of this family of proteins promote cellular fatty acid uptake and transport toward a specific metabolic pathway: esterification, β-oxidation, or fetal delivery across the syncytiotrophoblast BM. The cytoplasmic heart (FABP 3), liver (FABP 1) (9), adipose (FABP 4), and epidermal FABP (FABP 5) (5) isoforms have been shown to be expressed in primary trophoblasts and placental homogenates. The significance of the presence of several cytoplasmic FABP in trophoblasts is not yet understood, but their presence indicates that complex interactions of these proteins may be essential for efficient fatty acid transport and metabolism in the placenta.
Peroxisome proliferator-activated nuclear receptors (PPARs) act as lipid sensors. In particular, PPAR gamma (PPARγ) acts as a critical transcription factor in the regulation of lipid transport, storage, and metabolism (48). In the placenta, PPARγ regulates fatty acid transport in primary human trophoblasts (51) and in mice treated with PPARγ agonist rosiglitazone (52), indicating an important regulatory role of PPARγ in the uptake and placental transfer of lipids.
Increased fetal lipid availability may contribute to fetal overgrowth and/or increased fat accumulation in fetuses of obese mothers. Circulating levels of lipids (33, 48) are elevated in obese pregnant women compared with normal-weight pregnant women, which could promote fatty acid transfer across the placenta. In human placental homogenate, maternal obesity has been shown to decrease protein expression of FATP 4, whereas FAT/CD36 expression was increased (16). In rodent and bovine animal models of maternal obesity, the expression of several placental FATPs is increased (58, 61), suggesting an elevated placental capacity to transfer fatty acids. However, gene or protein expression of transporters in placental homogenate or whole tissue may not accurately reflect transport capacity, which is primarily determined by protein expression and/or activity of transporters localized to the two polarized plasma membranes of the syncytiotrophoblast.
In rodents, fetal exposure to maternal high-fat diet and insulin resistance during pregnancy results in obesity and hepatic lipid accumulation in the offspring (6, 28, 43, 57), leading to postnatal hepatic dysfunction and histologic features consistent with nonalcoholic steatohepatitis (6, 43). These findings suggest that the liver is a target for excess fat storage with intrauterine exposure to maternal overnutrition. Additionally, nonhuman primate models of maternal obesity exhibit increased fetal intrahepatic lipids that significantly accumulate during the third trimester (59). However, the mechanisms underlying the increased fetal hepatic fat deposition in maternal obesity are not well known, and whether an enhanced placental lipid transport capacity contributes to increased fetal lipid delivery is unknown.
In this study, we used a novel mouse model of maternal obesity associated with fetal overgrowth (50) to test the hypothesis that the expression of placental fatty acid transport-related proteins and fatty acid binding proteins is upregulated in response to maternal obesity, and this is associated with lipid accumulation in the liver of the offspring. We determined protein expression of FAT/CD36, FATP 2, 4, 6 in trophoblast plasma membranes isolated from the mouse placenta and FABPs 1, 3–5 in placental homogenates. In addition, we measured placental mRNA expression of lipid transport-related proteins and quantified lipid droplet accumulation in the fetal liver.
METHODS
Animals and diets.
All protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center San Antonio. Twelve-week-old C57/BL6J female mice (Jackson Laboratory, Bar Harbor, ME) were fed either a control (C; n = 10) diet (D12489, Research Diets, New Brunswick, NJ) containing 10% calories from fat or an obesogenic (OB; n = 10) diet (Western Diet D12089B, Research Diets), consisting of pellets containing 40% calories from fat and ad libitum access to 20% sucrose solution supplemented with micronutrients, vitamins (Vitamin Mix V10001, Research Diets), and minerals (Mineral Mix S10001, Research Diets), as previously described (50). When the females in the OB group had gained 25% of their initial body weight (after 4–6 wk on the obesogenic diet), the OB along with an equal number of C mice were pair-mated with males on the C diet. The presence of a postcopulatory plug indicated embryonic day (E) 0.5. All animals were maintained on their respective diets throughout gestation.
Tissue collection and processing.
At E18.5, dams were euthanized by carbon dioxide inhalation. After laparotomy, fetuses and placentas were collected and dried on blotting paper and weighed (50). All placentas in each litter (approximate total weight 0.5 g) were pooled and washed in PBS and transferred to 3 ml of buffer D (in mM: 250 sucrose, 1 Tris-HEPES, 1 EDTA, at pH 7.4). A protease and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) was added at a dilution of 1:1,000, and the mixture was homogenized using a Polytron (Kinematica, Bohemia, NY), frozen in liquid nitrogen, and stored at −80°C (homogenate) until immunoblotting analyses or isolation of trophoblast plasma membranes (TPM). Additionally, fetal livers (1 liver per litter, n = 14 per group) were dissected and weighed, placed onto a tissue mold, and filled with optimal cutting temperature (Fisher Scientific, Pittsburgh, PA) and frozen at −20°C. Using a cryostat, we obtained 10-μm-thickness fetal liver sections and stored them at −80°C.
Oil Red O staining.
An Oil Red O kit (Poly Scientific R&D, Bay Shore, NY) for staining neutral lipids was used (40). Frozen fetal liver parenchymal sections (2–3 per individual liver) were allowed to reach room temperature and placed in 70% ethanol for <5 s, then placed in Oil Red O solution for 25 min and rinsed; the nuclei was counterstained with Harris's hematoxylin for 1 min. Subsequently, sections were placed in a mixture 1% HCl-70% ethanol for 5 s. After rinsing, sections were mounted using glycerin jelly, and images were captured using a visible light microscope. Lipid droplets from fetal livers from C and OB dams were quantified using National Institutes of Health (NIH) ImageJ software (http://rsbweb.nih.gov/ij/).
Isolation of trophoblast layer II plasma membrane.
Maternal-facing trophoblast layer II plasma membrane (TPM) of the mouse placenta, which is believed to be functionally analogous to the human syncytiotrophoblast MVM (32, 51), was isolated using differential ultracentrifugation and Mg2+ precipitation (32, 51). Briefly, frozen placental homogenates were centrifuged at 10,000 g for 15 min, and the supernatant was retained and centrifuged at 125,000 g for 30 min. The resulting pellets were resuspended in buffer D, and 12 mM MgCl2 was added; this mixture was stirred for 15 min at 4°C and subsequently centrifuged at 2500 g for 10 min. The supernatant was spun at 125,000 g for 30 min, and the pellet was resuspended in buffer D and snap-frozen in liquid nitrogen prior to storing at −80°C. Protein concentrations of homogenate and isolated TPM were determined according to Lowry assay (DC protein assay; Bio-Rad, Hercules, CA). TPM enrichment was determined as the TPM/homogenate ratio of alkaline phosphatase activity, as previously described (19).
Lipase activity assay.
Placental lipase activity (lipoprotein and endothelial lipase) was measured in TPM from C and OB dams by the enzyme fluorescence method using a lipase activity assay kit (Roar Biomedical, New York, NY). A titration curve with increasing protein concentration of TPM and different incubation times at 37°C was initially performed (data not shown). TPM vesicles (10 μg protein) were incubated with the substrate solution for 30 min. Samples were read using a BioTek Synergy H1 Hybrid Reader at λex = 370 and λem = 450 nm, according to the manufacturer's instructions. Each sample was analyzed in duplicate, and the results were expressed as nanomoles of substrate hydrolyzed per 30 min.
Immunoblotting analysis.
Placental homogenate (20 μg) or TPM (5 μg) protein was separated by SDS-PAGE on precast gels (Bio-Rad) and transferred to polyvinylidene fluoride membranes (at 30V constant, overnight at 4°C). Blocking was carried out for 1 h at room temperature in 5% nonfat milk in Tris-buffered saline (TBS)-Tween, and membranes were incubated in primary antibody diluted in 1% BSA in TBS-Tween. Protein expression of FAT/CD36 and FATPs 2, 4, and 6 was determined in TPM, and FABPs 1, 3–5, total PPARγ, and (S112) pPPARγ were determined in homogenates. Primary antibodies were diluted as follows: rabbit polyclonal anti-FATP 2 at 3 μg/ml, anti-FAT/CD36, FATP 4, FATP 6, FABP 3, and FABP 5 at 1 μg/ml (AbCam, Cambridge, MA); anti-FABP 1 and FABP 4, PPARγ, (S112) pPPARγ at 1 μg/ml (Cell Signaling, Beverly, MA); and mouse anti-β-actin at 0.4 μg/ml (Sigma-Aldrich). Horseradish peroxidase-conjugated secondary anti-rabbit (0.5 μg/ml) and anti-mouse antibody (0.2 μg/ml) (Cell Signaling) were used. Target protein expression was normalized to β-actin expression. Immunolabeling was visualized with enhanced chemiluminescent detection solution (Thermo Scientific, Waltham, MA) and a G:BOX Chemi XT4 gel imaging system (Syngene, Cambridge, UK). Densitometry was performed using NIH's ImageJ software. After normalization to β-actin, the mean density of the C group bands for each protein target was assigned an arbitrary value of 1. Subsequently, all individual C and OB density values were expressed relative to this mean.
Reverse transcription and quantitative PCR.
Total RNA was extracted from placental homogenates (25–75 mg/litter) with TRIzol Reagent (Life Technologies, Carlsbad, CA) following the manufacturer's protocol. The RNA 280/260 ratio ranged from 1.8 to 2.1. cDNA was synthesized using the high-capacity RNA-to-cDNA kit (Life Technologies). Quantitative PCR was performed in triplicate on 0.2 μg of total RNA reverse transcribed into cDNA using SYBR select master mix (Life Technologies). PCR amplification and detection were performed on a Quant Studio 6 Flex real-time PCR system (Life Technologies) using the gene-specific primers listed in Table 1. Amplification of a single product was confirmed by melting curve analysis. The amplified transcripts were quantified using the relative standard curve method and normalized to the geometric mean of housekeeping genes Gadph (encoding glyceraldehyde 3-phosphate dehydrogenase) and Trfc (encoding transferrin receptor).
Table 1.
Sequences for real-time quantitative PCR primers
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (3′ to 5′) |
|---|---|---|
| Fat/Cd36 | CCAGTGTATATGTAGGCTCATCCA | TGGCCTTACTTGGGATTGG |
| Fatp2 | GCTGACATCGTGGGACTGGT | TTCGACCCTCATGACCTGGC |
| Fatp4 | GGCTTCCCTGGTGTACTATGGAT | ACGATGTTTCCTGCTGAGTGGTA |
| Fatp6 | GGCTTGAGGATGCCGCTTA | GTACTCTGGGCTCATGCTATGAAGT |
| Fabp1 | GTGACTGAACTCAATGGAGACAC | GTAGACAATGTCGCCCAATGTCA |
| Fabp3 | CATGAAGTCACTCGGTGTGG | TGCCATGAGTGAGAGTCAGG |
| Fabp4 | AAGAAGTGGGAGTGGGCTTT | TCGACTTTCCATCCCACTTC |
| Fabp5 | AGAGCACAGTGAAGACGAC | CATGACACACTCCACGATCA |
| Pparγ | TGTGGGGATAAAGCATCAGGC | CCGGCAGTTAAGATCACACCTAT |
| Lpl | AATTTGCTTTCGATGTCTGAGAA | CAGAGTTTGACCGCCTTCC |
| Ldhb | CATTGCGTCCGTTGCAGATG | GGAGGAACAAGCTCCCGTG |
| Dgat1 | TTTGCTCTGGCATCATACTCC | CCACTGACCTTCTTCCCTGTA |
| Dcakd | CCGAGGCTACCGATACGTGA | GTTCAGGTTGTTCCGCTTCAT |
| C/Ebpα | TGGACAAGAACAGCAACGAGTAC | GCAGTTGCCCATGGCCTTGAC |
| Srebp1c | AGACAAACTGCCCATCCAC | AAGCGGATGTAGTCGATGGC |
| Gadph | AGTGGCAAAGTGGAGATT | GTGGAGTCATACTGGAACA |
| Trfc | GTTTCTGCCAGCCCCTTATTAT | GCAAGGAAAGGATATGCAGCA |
Data presentation and statistical analysis.
Data are presented as means ± SE or + SE; n represents number of litters. Statistical analysis and plotting were performed using GraphPad Prism version 6 (GraphPad Software, La Jolla, CA). Gaussian distribution of the samples was determined by D'Agostino and Pearson omnibus normality test, and statistical significance between C and OB groups was determined by Student's unpaired t-test. A P value less than 0.05 was considered statistically significant.
RESULTS
TPM alkaline phosphatase enrichment was similar between C and OB groups.
Alkaline phosphatase is a specific marker of the trophoblast layer II of the mouse placenta (32), and the TPM/homogenate ratio of alkaline phosphatase activity is, therefore, used as measurement of TPM enrichment. The average alkaline phosphatase enrichment for TPM vesicles isolated from C placentas (n = 10) was 11.05 ± 0.91, comparable to the enrichment in TPM vesicles obtained from placentas of OB animals 10.63 ± 0.65 (n = 10; no significant difference between groups, Student's unpaired t-test).
Placental FATP 6 expression was increased in TPM from OB dams.
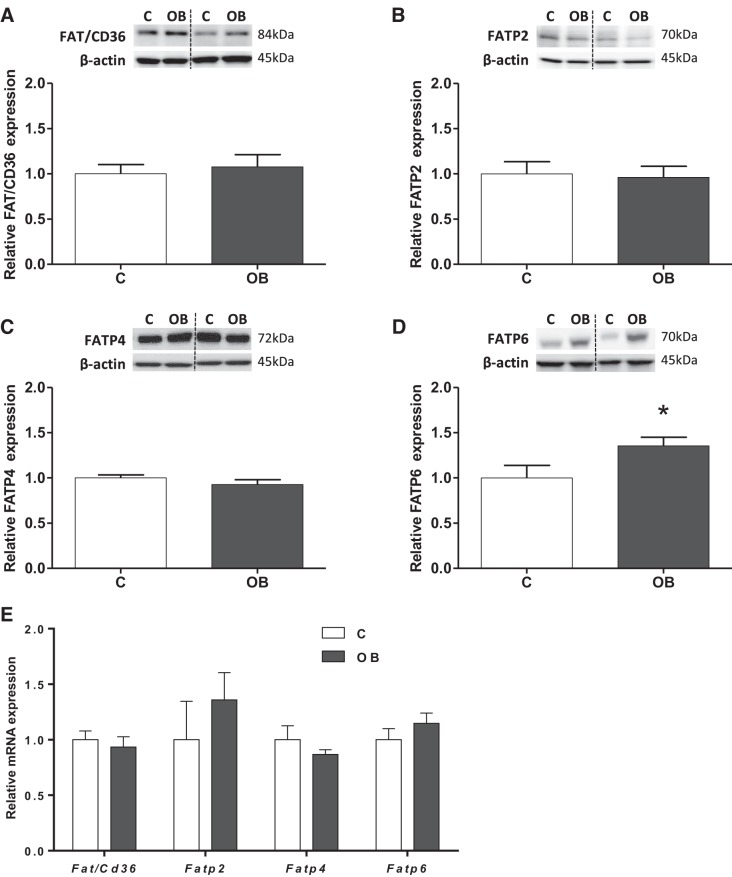
Protein expression of fatty acid transport-related proteins FAT/CD36, FATP 2, FATP 4, and FATP 6 was assessed in purified mouse placental TPM. FAT/CD36, FATP 2, and FATP 4 proteins were expressed in TPM, and their expression was comparable between placentas from C and OB dams (Fig. 1, A–C). Notably, TPM FATP 6 protein expression was increased 35% (P < 0.05) in OB compared with C placentas (Fig. 1D). In addition, we evaluated the mRNA expression of Fatp/Cd36, Fatp 2, Fatp 4, and Fatp 6; however, the expression was unchanged in placentas from OB compared with C dams (Fig. 1E).
Fig. 1.
Expression of fatty acid translocase (FAT)/CD36 and fatty acid transport protein (FATP) 2, FATP 4, and FATP 6 in trophoblast plasma membrane (TPM) from control (C) and obese (OB) dams. Representative immunoblots with corresponding histograms illustrating relative protein expression of FAT/CD36 (A), FATP 2 (B), FATP 4 (C), and FATP 6 (D). E: placental mRNA expression of Fat/Cd36, Fatp 2, 4, and 6 normalized to the geometric mean of Gadph and Tfrc mRNA. Data are expressed as means + SE; n = 10/group. Statistical significance was determined using Student's unpaired t-test, *P < 0.05.
Placental lipase activity and PPARγ expression were unaffected by maternal obesity.
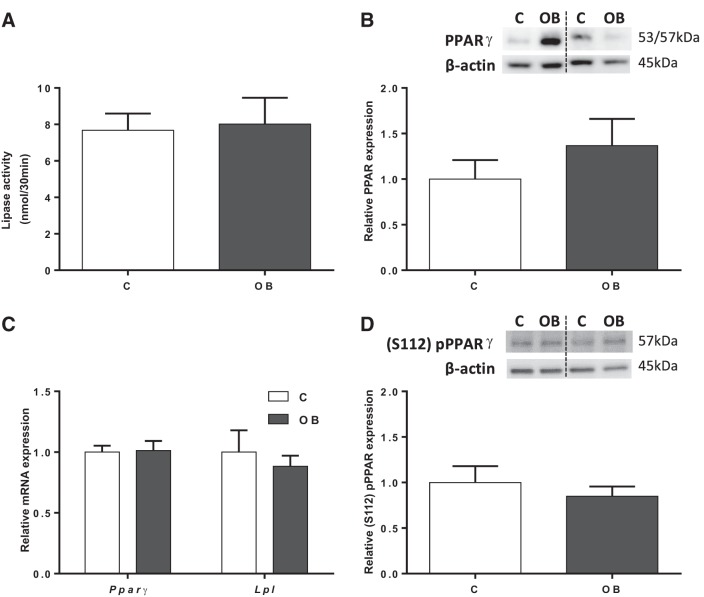
Placental lipase activity was determined in isolated TPM from placentas from C and OB dams. Lipase activity was comparable between C and OB groups (Fig. 2A). Furthermore, protein expression of PPARγ, a master regulator of lipid metabolism that modulates fatty acid transport in the placenta (53), was assessed in mouse placental homogenates. Total PPARγ protein expression did not change between groups (Fig. 2B). In addition, mRNA expression of both Pparγ and Lpl was unchanged in placental homogenates from OB compared with C dams (Fig. 2C). PPARγ can be activated by ligand-independent mechanisms, in particular, by phosphorylation at serine-112 [(S112) pPPARγ] (2, 8). However, S112 phosphorylation of PPARγ in placental homogenates was not significantly different between C and OB groups (Fig. 2D).
Fig. 2.
Activity and expression of regulators of placental fatty acid uptake. A: lipase activity in TPM from C and OB dams. B: representative immunoblots with corresponding histogram illustrating relative placental protein expression of PPARγ in homogenate from C and OB dams. C: placental mRNA expression of Pparγ and Lpl normalized to the geometric mean of Gadph and Tfrc mRNA. D: representative immunoblots with corresponding histogram illustrating relative placental protein expression of (S112) pPPARγ in homogenate from C and OB dams. Values are expressed as means + SE; n = 10/group. Statistical significance was determined using Student's unpaired t-test.
Expression of PPARγ-regulated genes was unaltered by maternal obesity.
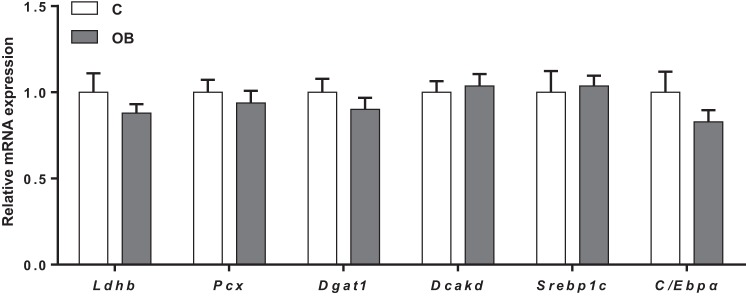
We further explored the expression of PPARγ-regulated genes in placental homogenates from C and OB dams. mRNA of Ldhb (encoding for lactate dehydrogenase), Pcx (pyruvate carboxylase), Dgat1 (diacylglycerol O-acyltransferase-1), Dcakd (dephospho-CoA kinase domain containing) (56), as well as key lipogenic factors C/Ebpα (CCAAT/enhancer binding protein-α) and Srebp1c (sterol regulatory element-binding protein-1c), all PPARγ transcriptional targets involved in intermediary metabolism, were expressed in placental homogenates from C and OB dams; however, the expression did not differ between groups (Fig. 3).
Fig. 3.
Placental mRNA expression of PPARγ-regulated genes and other transcription factors involved in lipid metabolism. mRNA expression of Ldhb, Pcx, Dgat1, Dcakd, Srebp1c, and C/Ebpα in C and OB dams normalized to the geometric mean of Gadph and Tfrc mRNA. Values are expressed as means + SE; n = 10/group. Statistical significance was determined using Student's unpaired t-test.
Placental FABP 3 expression was increased in OB dams.
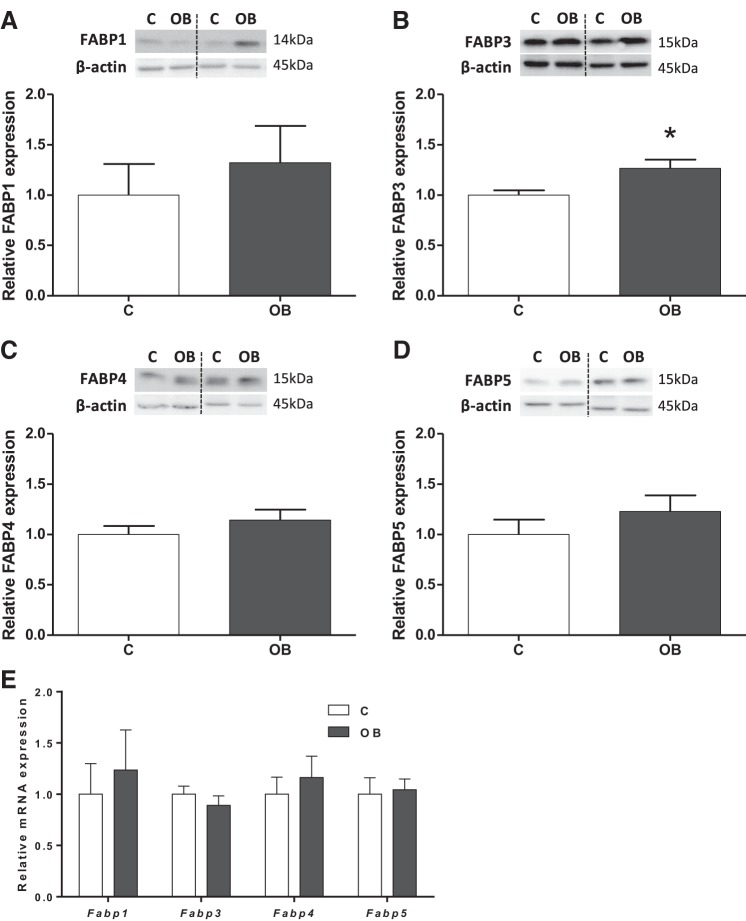
Protein expression of fatty acid binding proteins FABPs 1, 3–5 was assessed in mouse placental homogenates. FABPs 1, 4–5 proteins and mRNA were expressed in homogenates, and their levels were comparable between placentas from C and OB dams (Fig. 4, A, C–E). In contrast, FABP 3 expression was increased 27% (P < 0.01) in OB compared with C placentas (Fig. 4B), without changes in mRNA levels (Fig. 4E).
Fig. 4.
Placental expression of FABPs 1, 3–5 in homogenate from C and OB dams. Representative immunoblots with corresponding histograms illustrating relative protein expression of FABP 1 (A), FABP 3 (B), FABP 4 (C) and FABP 5 (D). E: placental mRNA expression of Fabps 1, 3–5 normalized to the geometric mean of Gadph and Tfrc mRNA. Data are expressed as means + SE; n = 10/group. Statistical significance was determined using Student's unpaired t-test, *P < 0.01.
Maternal obesity increased lipid accumulation in the fetal liver.
In the same cohort of mice studied in the current report, we have previously established that fetal weight at E18.5 in the OB group was significantly increased by 18% (P < 0.01) compared with fetuses from C dams, without affecting placental weight (50). This was not due to a difference in litter size, which was comparable in the C and OB groups (50). Livers from OB fetuses were 31% heavier (52.1 ± 4.85 mg; n = 6) compared with livers from fetuses from the C group (39.7 ± 2.35 mg; n = 7; mean ± SE; P < 0.05; Student's unpaired t-test). In addition, fetal liver lipid accumulation was determined using Oil Red O staining. The number of lipid droplets were increased 10-fold in the livers of fetuses from OB compared with C group (P < 0.05) (Fig. 5).
Fig. 5.
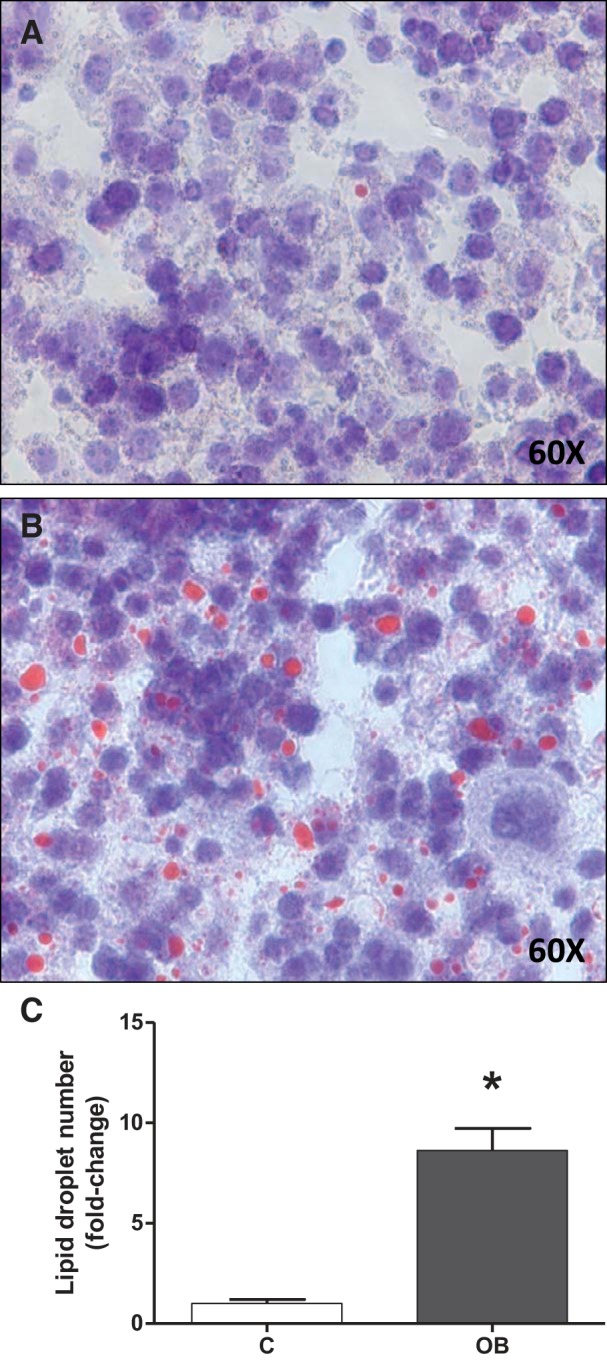
Lipid accumulation in fetal livers. Representative sections from fetal liver from C (A) and OB (B) dams stained for Oil Red O. C: corresponding histogram illustrating fetal liver lipid droplet number. Values are expressed as means + SE; n = 14/group. Statistical significance was determined using Student's unpaired t-test, *P < 0.05.
DISCUSSION
We report, for the first time, that FATP 6 and FABP 3 expression is elevated in the placental barrier of obese dams, suggesting that increased placental lipid transfer capacity could contribute to fetal overgrowth and greater lipid accumulation in the fetal liver.
Our results show that TPM isolated from mouse placentas from C and OB dams were enriched 10-fold in alkaline phosphatase activity, confirming the successful isolation of this trophoblast apical membrane fraction. These results are in agreement with previous reports that showed alkaline phosphatase staining was localized to the apical (maternal facing) plasma membrane of trophoblast layer II in the mouse (32), analogous to alkaline phosphatase localized to the human syncytiotrophoblast MVM (30). Plasma membrane transporters contribute to cellular uptake only if present in the cell surface membrane, and placental transporters mediate transplacental transport only if localized in the plasma membranes of the cells believed to form the placental barrier. Thus, our study of transporter protein expression in TPM is potentially more informative than previous reports of gene and protein expression of fatty acid transporters in placental homogenates in obese pregnant women (16) and in animal experimental models (58, 61).
FATP family members are regulated by hormones (e.g., insulin), inflammatory mediators, such as TNF-α and IL-1, and by activators of PPARα or PPARγ (21, 52). Previous studies have demonstrated mRNA expression of members of the fatty acid transport family (FATPs 1–4, and 6), FAT/CD36 at E18.5 (53), and of FATP 4 at the protein level at E17.5 in the murine placenta (41). We now extend these observations by demonstrating specific expression of FATPs in TPM isolated from the mouse placenta. A maternal high-fat, high-sugar obesogenic diet led to greater placental expression of FATP 6 compared with dams on a control diet, suggesting that increased protein expression of this fatty acid transport protein in obesity could promote greater fatty acid transfer to the fetus. In addition, protein expression of FATP 6 in TPM from OB placentas was not associated with increased mRNA levels, suggesting that the change in protein level occurs at a posttranscriptional level, in agreement with similar changes reported for other transporter proteins (23). Little is currently known about the function of FATP 6. It is the predominant FATP expressed in the heart (22) and other tissues (4, 54). In cardiac myocytes (sarcolemma) FATP 6 mediates the uptake of long-chain fatty acids (palmitic acid > oleic acid > linoleic acid) (22). Additionally, in a rat model of myocardial infarction, FATP 6 protein levels were significantly reduced, which was associated with a decrease in fatty acid uptake (25). Conversely, it is still unclear which factors modulate FATP 6 expression in a maternal obesogenic environment. Although rosiglitazone, a PPARγ agonist and positive upstream modulator of the expression of FATP 1 and FATP 4 in the murine placenta (E18.5), increased FATP 6 mRNA expression (53), placental PPARγ signaling was not significantly activated in the placenta of obese dams. In addition, although maternal insulin and leptin levels in our mouse model of maternal obesity were significantly increased (50), currently, there is no evidence of hormonal regulation of FATP 6. Thus, further studies are required to identify the signaling pathways causing the upregulation of placental FATP 6 in maternal obesity.
Fatty acids are known to be positive upstream regulators of PPARγ (60), which is a critical regulator of fatty acid uptake in the placenta (52, 53). However, we found no evidence of activation of PPARγ in the placenta of obese dams, because placental protein expression and phosphorylation of PPARγ and the mRNA expression of PPARγ-downstream regulated genes were unaltered in maternal obesity. These findings are consistent with our previous observations that maternal obesity is not associated with significant changes in circulating levels of TAG or nonesterified fatty acids in our mouse model (50). Our findings are in contrast to Qiao et al. (47), who reported that maternal high-fat diet feeding in mice is associated with increased expression of PPARγ in the placenta (47). Furthermore, in this mouse model, placental lipase activity was increased in response to a high-fat diet, which is consistent with one report linking maternal obesity in pregnant women to elevated placental lipase activity (16). Because the expression of placental lipases may be regulated by PPARγ (47), the unchanged placental lipase mRNA expression and activity in obese dams in our study is consistent with unaffected placental PPARγ signaling.
Our data corroborate previously reported expression of FABPs 3–5 (29, 38) in the murine placenta at E18.5. In addition, we demonstrated that FABP 1 is expressed at the mRNA and protein levels in the placenta of the mouse. FABPs are believed to participate in a variety of metabolic functions in the placenta. Specifically, FABP 4 is involved in TAG accumulation (38), and placenta-specific FABP 3-knockout results in impaired placental long-chain fatty acid trafficking with lower fetal accumulation of long-chain fatty acids (29). In our study, placental FABP mRNA levels were unaffected by maternal obesity. These results are in general agreement with Dubé et al. (16), who reported unchanged FABP3 mRNA expression in placentas of obese pregnant women. We propose that the elevated FABP 3 expression in placentas from OB compared with C dams could promote increased transplacental fatty acid transport in maternal obesity.
Infants born to obese mothers have increased liver fat compared with infants of lean mothers (7). This is replicated in our mouse model of obesity because lipid droplet number was markedly increased in fetuses of obese dams compared with their lean counterparts in agreement with previous animal experimental data (6, 28, 43, 57). The increased lipid accumulation in the fetal liver in response to maternal obesity may, in part, be caused by enhanced placental lipid transfer, as indicated by the demonstration of an elevated placental lipid transport capacity in obese dams in our study. However, other mechanisms may also be involved in the development of fetal liver NAFLD, such as dysregulation of the de novo lipogenic capacity favoring lipid synthesis (59). Moreover, lipids activate different cell signaling pathways; therefore, an elevated lipid exposure in utero could potentially alter gene expression in the fetal liver (26). Given the pronounced fetal hyperglycemia in our mouse obesity model (50), it is also possible that the combination of hyperglycemia and insulin concentrations (hyperinsulinemia) promotes de novo fatty acid synthesis (lipogenesis) and impairs β-oxidation, thereby contributing to the development of fetal hepatic steatosis (46). On the other hand, in high-fat fed nonhuman primates, the expression of genes involved in de novo lipogenesis in fetal livers remained unchanged, and de novo lipid synthesis was negligible compared with fetuses of mothers fed a control diet (39). This indicates that de novo lipogenesis is not an important contributor to fat accumulation in fetal liver in animals fed a high-fat diet.
Perspectives and Significance
Obese women often deliver infants that are large at birth and/or have increased adiposity; however, the underlying mechanisms are not well established. We report that the protein expression of FATP 6 and FABP 3 is increased in the placental barrier in a mouse model of obesity in pregnancy, and we propose that this enhanced capacity of the placenta to transport fatty acids contributes to lipid accumulation and fetal overgrowth in obesity. These findings are consistent with the emerging concept that changes in the maternal environment, such as a high-fat diet and obesity, impact the fetus, mediated by changes in placental function.
GRANTS
This study was supported National Institutes of Health Grant R24OD016724.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.D., F.J.R., T.L.P., and T.J. conception and design of research; P.D., J.H., and F.J.R. performed experiments; P.D. and J.H. analyzed data; P.D., F.J.R., T.L.P., and T.J. interpreted results of experiments; P.D. prepared figures; P.D. drafted manuscript; P.D., T.L.P., and T.J. edited and revised manuscript; P.D., J.H., F.J.R., T.L.P., and T.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge the help of Irving L. M. H. Aye for excellent technical assistance.
REFERENCES
- 1.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 268: 17,665–17,668, 1993. [PubMed] [Google Scholar]
- 2.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 272: 5128–5132, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet 2: 27, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bionaz M, Loor JJ. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 are the most abundant isoforms in bovine mammary tissue and their expression is affected by stage of lactation. J Nutr 138: 1019–1024, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Biron-Shental T, Schaiff WT, Ratajczak CK, Bildirici I, Nelson DM, Sadovsky Y. Hypoxia regulates the expression of fatty acid-binding proteins in primary term human trophoblasts. Am J Obstet Gynecol 197: 516 e511–e516, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA, McConnell JM, Byrne CD. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50: 1796–1808, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, Reynolds R, Alston M, Hoffman C, Pan Z, Friedman JE, Barbour LA. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr 162: 930-6.e1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology 140: 392–397, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Campbell FM, Bush PG, Veerkamp JH, Dutta-Roy AK. Detection and cellular localization of plasma membrane-associated and cytoplasmic fatty acid-binding proteins in human placenta. Placenta 19: 409–415, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Campbell FM, and Dutta-Roy AK. Plasma membrane fatty acid-binding protein (FABPpm) is exclusively located in the maternal facing membranes of the human placenta. FEBS Lett 375: 227–230, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Campbell FM, Taffesse S, Gordon MJ, Dutta-Roy AK. Plasma membrane fatty-acid-binding protein in human placenta: identification and characterization. Biochem Biophys Res Commun 209: 1011–1017, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32: 1076–1080, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darmady JM, Postle AD. Lipid metabolism in pregnancy. Br J Obstet Gynaecol 89: 211–215, 1982. [DOI] [PubMed] [Google Scholar]
- 14.Diaz P, Powell TL, Jansson T. The role of placental nutrient sensing in maternal-fetal resource allocation. Biol Reprod 91: 82, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilworth MR, Sibley CP. Transport across the placenta of mice and women. Placenta 34 Suppl: S34–S39, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Dubé E, Gravel A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, Forest JC, Giguere Y, Masse A, Lafond J. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod 87: 1–11, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Dutta-Roy AK. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am J Clin Nutr 71: 315S–322S, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Enders AC, Blankenship TN. Comparative placental structure. Adv Drug Deliv Rev 38: 3–15, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Gaccioli F, White V, Capobianco E, Powell TL, Jawerbaum A, Jansson T. Maternal overweight induced by a diet with high content of saturated fat activates placental mTOR and eIF2alpha signaling and increases fetal growth in rats. Biol Reprod 89: 1–11, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Gauster M, Hiden U, Blaschitz A, Frank S, Lang U, Alvino G, Cetin I, Desoye G, Wadsack C. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab 92: 2256–2263, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Gimeno RE. Fatty acid transport proteins. Curr Opin Lipidol 18: 271–276, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Gimeno RE, Ortegon AM, Patel S, Punreddy S, Ge P, Sun Y, Lodish HF, Stahl A. Characterization of a heart-specific fatty acid transport protein. J Biol Chem 278: 16,039–16,044, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci USA 105: 17,402–17,407, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haggarty P. Fatty acid supply to the human fetus. Ann Rev Nutrit 30: 237–255, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Heather LC, Cole MA, Lygate CA, Evans RD, Stuckey DJ, Murray AJ, Neubauer S, Clarke K. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc Res 72: 430–437, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol 299: R711–R722, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera E, Ortega-Senovilla H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr Pharmaceut Biotechnol 15: 24–31, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Isganaitis E, Woo M, Ma H, Chen M, Kong W, Lytras A, Sales V, Decoste-Lopez J, Lee KJ, Leatherwood C, Lee D, Fitzpatrick C, Gall W, Watkins S, Patti ME. Developmental programming by maternal insulin resistance: hyperinsulinemia, glucose intolerance, and dysregulated lipid metabolism in male offspring of insulin-resistant mice. Diabetes 63: 688–700, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islam A, Kagawa Y, Sharifi K, Ebrahimi M, Miyazaki H, Yasumoto Y, Kawamura S, Yamamoto Y, Sakaguti S, Sawada T, Tokuda N, Sugino N, Suzuki R, Owada Y. Fatty acid binding protein 3 is involved in n-3 and n-6 PUFA transport in mouse trophoblasts. J Nutr 144: 1509–1516, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Jones CJ, Fox H. An ultrahistochemical study of the distribution of acid and alkaline phosphatases in placentae from normal and complicated pregnancies. J Pathol 118: 143–151, 1976. [DOI] [PubMed] [Google Scholar]
- 31.Kaminsky S, Sibley CP, Maresh M, Thomas CR, D'Souza SW. The effects of diabetes on placental lipase activity in the rat and human. Pediatr Res 30: 541–543, 1991. [DOI] [PubMed] [Google Scholar]
- 32.Kusinski LC, Jones CJ, Baker PN, Sibley CP, Glazier JD. Isolation of plasma membrane vesicles from mouse placenta at term and measurement of system A and system beta amino acid transporter activity. Placenta 31: 53–59, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lager S, Gaccioli F, Ramirez VI, Jones HN, Jansson T, Powell TL. Oleic acid stimulates system A amino acid transport in primary human trophoblast cells mediated by Toll-like receptor 4. J Lipid Res 54: 725–733, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larque E, Demmelmair H, Klingler M, De Jonge S, Bondy B, Koletzko B. Expression pattern of fatty acid transport protein-1 (FATP-1), FATP-4 and heart-fatty acid binding protein (H-FABP) genes in human term placenta. Early Hum Dev 82: 697–701, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Larque E, Krauss-Etschmann S, Campoy C, Hartl D, Linde J, Klingler M, Demmelmair H, Cano A, Gil A, Bondy B, Koletzko B. Docosahexaenoic acid supply in pregnancy affects placental expression of fatty acid transport proteins. Am J Clin Nutr 84: 853–861, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Larque E, Ruiz-Palacios M, Koletzko B. Placental regulation of fetal nutrient supply. Curr Opin Clin Nutr Metab Care 16: 292–297, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity—a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol 8: 679–688, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Makkar A, Mishima T, Chang G, Scifres C, Sadovsky Y. Fatty acid binding protein-4 is expressed in the mouse placental labyrinth, yet is dispensable for placental triglyceride accumulation and fetal growth. Placenta 35: 802–807, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119: 323–335, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc 8: 1149–1154, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Mishima T, Miner JH, Morizane M, Stahl A, Sadovsky Y. The expression and function of fatty acid transport protein-2 and -4 in the murine placenta. PLoS One 6: e25865, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modi N, Murgasova D, Ruager-Martin R, Thomas EL, Hyde MJ, Gale C, Santhakumaran S, Dore CJ, Alavi A, Bell JD. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res 70: 287–291, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, Soeda J, Fernandez-Twinn DS, Martin-Gronert MS, Ozanne SE, Sigala B, Novelli M, Poston L, Taylor PD. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol 52: 913–920, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 1–8, 2012. [PubMed] [Google Scholar]
- 45.Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect 108 Suppl 3: 545–553, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 118: 829–838, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao L, Guo Z, Bosco C, Guidotti S, Wang Y, Wang M, Parast M, Schaack J, Hay WW, Moore TR, Shao J. Maternal high-fat feeding increases placental lipoprotein lipase activity by reducing SIRT1 expression in mice. Diabetes 64: 3111–3120, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 87: 4231–4237, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Rogue A, Spire C, Brun M, Claude N, Guillouzo A. Gene expression changes induced by PPAR gamma agonists in animal and human liver. PPAR Res 2010: 325183, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity (Silver Spring) 23: 1663–1670, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosario FJ, Schumacher MA, Jiang J, Kanai Y, Powell TL, Jansson T. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol 590: 1495–1509, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaiff WT, Bildirici I, Cheong M, Chern PL, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab 90: 4267–4275, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Schaiff WT, Knapp FF Jr, Barak Y, Biron-Shental T, Nelson DM, Sadovsky Y. Ligand-activated peroxisome proliferator activated receptor gamma alters placental morphology and placental fatty acid uptake in mice. Endocrinology 148: 3625–3634, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Schmuth M, Ortegon AM, Mao-Qiang M, Elias PM, Feingold KR, Stahl A. Differential expression of fatty acid transport proteins in epidermis and skin appendages. J Invest Dermatol 125: 1174–1181, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 195: 1100–1103, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Shalom-Barak T, Zhang X, Chu T, Timothy Schaiff W, Reddy JK, Xu J, Sadovsky Y, Barak Y. Placental PPARgamma regulates spatiotemporally diverse genes and a unique metabolic network. Dev Biol 372: 143–155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 294: R528–R538, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Strakovsky RS, Pan YX. A decrease in DKK1, a WNT inhibitor, contributes to placental lipid accumulation in an obesity-prone rat model. Biol Reprod 86: 81, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorn SR, Baquero KC, Newsom SA, El Kasmi KC, Bergman BC, Shulman GI, Grove KL, Friedman JE. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes 63: 2702–2713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 1812: 1007–1022, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu MJ, Ma Y, Long NM, Du M, Ford SP. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol 299: R1224–R1231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]