Summary
Background
Although the association between psychotic illness and cigarette smoking is well known, the reasons are unclear why people with psychosis are more likely to smoke than are the general population. We aimed to test several hypotheses. First, that daily tobacco use is associated with an increased risk of psychotic illness in both case-control and prospective studies. Second, that smoking is associated with an earlier age at onset of psychotic illness. Finally, that an earlier age at initiation of smoking is associated with an increased risk of psychosis. We also aimed to derive an estimate of the prevalence of smoking in patients presenting with their first episode of psychosis.
Methods
We searched Embase, Medline, and PsycINFO and selected observational studies in which rates of smoking were reported in people with psychotic disorders, compared with controls. We calculated the weighted mean difference for age at onset of psychosis and age at initiation of smoking. For categorical outcomes, we calculated odds ratios from cross-sectional studies and risk ratios from prospective studies.
Findings
Of 3717 citations retrieved, 61 studies comprising 72 samples met inclusion criteria. The overall sample included 14 555 tobacco users and 273 162 non-users. The prevalence of smoking in patients presenting with their first episode of psychosis was 0·57 (95% CI 0·52–0·62; p<0·0001). In case-control studies, the overall odds ratio for the first episode of psychosis in smokers versus non-smokers was 3·22 (95% CI 1·63–6·33), with some evidence of publication bias (Egger's test p=0·018, Begg's test p=0·007). For prospective studies, we calculated an overall relative risk of new psychotic disorders in daily smokers versus non-smokers of 2·18 (95% CI 1·23–3·85). Daily smokers developed psychotic illness at an earlier age than did non-smokers (weighted mean difference −1·04 years, 95% CI −1·82 to −0·26). Those with psychosis started smoking at a non-significantly earlier age than did healthy controls (−0·44 years, 95% CI −1·21 to 0·34).
Interpretation
Daily tobacco use is associated with increased risk of psychosis and an earlier age at onset of psychotic illness. The possibility of a causal link between tobacco use and psychosis merits further examination.
Funding
NIHR Maudsley Biomedical Research Centre.
Introduction
Although the association between smoking tobacco and psychosis (in particular, schizophrenia) has been acknowledged,1 the reasons why people with psychosis are more likely to smoke compared with the rest of the population are still unclear. Several theories have been proposed, many focusing on the idea of self-medication—ie, smoking corrects a pharmacological abnormality (such as excessive dopamine blockade induced by antipsychotics), counteracts negative or cognitive symptoms of schizophrenia,2 or relieves boredom or distress. Little attention has been directed towards the possibility that cigarette smoking might increase risk for the disorder.3 This shortfall is surprising, particularly in view of the large amount of attention paid to the role of other substances of misuse (notably cannabis and stimulants) in the aetiology of psychotic illness.4, 5, 6, 7 This discrepancy is exemplified by two meta-analyses: in the first, 83 studies were included in which the onset of psychosis and cannabis use was analysed;8 in the second, ten studies investigated tobacco use and onset of psychotic illness.9
In the second meta-analysis,9 compared with healthy controls, an increased risk of tobacco smoking was noted in people who developed psychosis (odds ratio 6·04, 95% CI 3·03–12·02), with no difference in age at onset of psychosis between smokers and non-smokers (standardised mean difference −0·03).10 However, since that review was published in 2012, several newer studies have become available, with additional data for daily tobacco use.11, 12, 13, 14, 15 Furthermore, standardised units were used in the 2012 meta-analysis in place of years for age at onset, which was not necessary because all studies used the same units (years).
We undertook a systematic review and meta-analysis of prospective, case-control, and cross-sectional studies to test four hypotheses. First, that an excess of tobacco use is already present in people presenting with their first episode of psychosis. Second, that daily tobacco use is associated with an increased risk of subsequent psychotic disorder. Third, that daily tobacco use is associated with an earlier age at onset of psychotic illness. Fourth, that an earlier age at initiation of smoking is associated with an increased risk of psychotic disorder. We aimed to produce a weighted mean difference in age at onset, expressed in years. Finally, we aimed to estimate the prevalence of smoking in people presenting with their first episode of psychosis.
Methods
Systematic review
We did a systematic review in accordance with MOOSE16 and PRISMA17 guidelines. We searched Embase (from 1980 to 2014 [week 4]), Medline (1980 to 2014 [week 4]), and PsycINFO (from 1980 to 2014 [week 3]) with the search terms “schizophrenia” OR “schizo*” OR “psychosis” AND “nicotine” OR “smoking” OR “cigarette smoking”, with no language restriction. We screened the abstracts of articles and retrieved the full text of relevant studies; we also checked reference lists and citation histories. Two of us (PG and SJ) selected studies.
We included studies that used ICD or DSM criteria (from DSM III and ICD-8 onwards) for psychotic disorders: schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder, non-affective psychotic disorder, atypical psychosis, psychotic depression, and bipolar mania with psychotic features. To assess the prevalence of smoking in patients presenting with their first episode of psychotic illness, we included studies in which the rates of smoking were reported for people having contact with secondary mental health services for the first time. For our first hypothesis, we included studies that also contained a control group, so we could calculate odds ratios in addition to prevalence. For our second hypothesis, we included prospective studies in which rates of smoking (daily tobacco use) were reported for patients who developed or had psychotic disorders compared with controls, enabling calculation of risk ratios. For our third and fourth hypotheses, we included prospective and case-control studies, and for the onset of psychosis we also included cross-sectional studies.
We excluded studies on the basis of insufficient data or if they met our exclusion criteria. These criteria were a primary focus on people with substance-induced psychosis, organic psychosis, or learning disability.
Data collection
We extracted data according to a specific protocol. First, we identified the proportion of smokers and non-smokers with a diagnosed first episode of psychosis. Second, we ascertained the risk of psychosis in daily smokers and non-smokers from longitudinal prospective studies. Third, we established the mean (with SD) age at onset of psychotic illness for daily smokers and non-smokers. Finally, we looked at initiation of tobacco smoking in patients diagnosed with psychotic illness versus controls, comparing smokers with non-smokers. For every study, we extracted specific data to include in the model: author, country, and year of publication; type of study (ie, prospective, cross-sectional, case-control, retrospective); demographic characteristics (eg, sex, age); clinical assessments (ie, diagnostic criteria, disease diagnosed); cannabis and other substances of misuse (ie, alcohol, caffeine, cocaine).
Definitions
We identified self-reported current tobacco use in the included studies. Our preferred term was “daily tobacco use”, which we defined as either cigarettes smoked per day or by use of the term “regular smoking”, or a Fagerström Test for Nicotine Dependence score. If the study used terms such as “current smoker” or “smoker” without stipulating the number of cigarettes or packs smoked per day, we did not judge tobacco use to be daily and we did not include the study in the analysis of daily smokers.
We defined onset of psychotic illness as either first psychiatric inpatient care,18, 19, 20, 21, 22, 23 start of medical treatment,24 the first episode of psychosis,19, 20, 22, 23, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 or the first diagnosis of psychotic illness.18, 19, 21, 24, 26, 27, 28, 30, 33, 40, 41, 42, 43, 44, 45, 46 Because the definitions of age at onset differed, we had to assume that the difference in age at onset between smokers and non-smokers was similar, irrespective of the definition used.3 For the same reason, we used a random-effects model.
Statistical analysis
We calculated the weighted mean difference for continuous data, which were age at onset of psychosis and age at initiation of smoking. For the categorical outcomes of rates of tobacco use in patients and controls, we calculated odds ratios from cross-sectional data and relative risks from prospective data.
We used Stata version 10 with the metan command. We judged a p value less than 0·05 significant. We used a random-effects model in all analyses because we expected the data to be heterogeneous across studies. We calculated I2 values to test for heterogeneity between studies. We deemed I2 less than 25% to have low heterogeneity, 25–75% to have medium heterogeneity, and greater than 75% to have high heterogeneity. We also assessed publication bias and selective reporting with Egger's and Begg's tests47, 48 and by inspecting the symmetry of funnel plots, as recommended in the Cochrane handbook.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
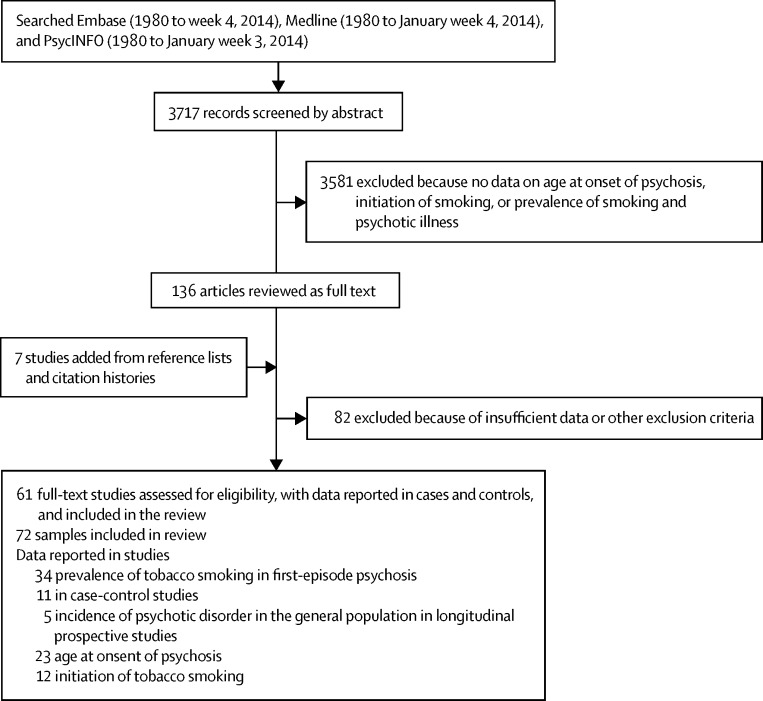
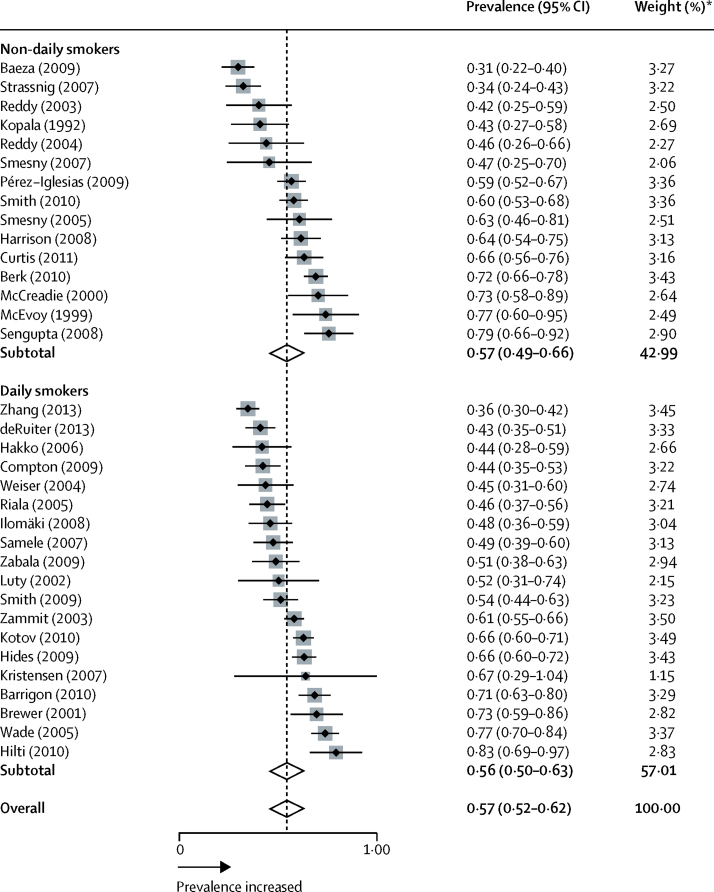
61 studies comprising 72 samples were analysed (figure 1; appendix pp 1–2). 7475 people with psychosis were smokers (mean 33·2 per sample [SD 195·7]) and 5670 people with psychosis were non-smokers (mean 20·7 per sample [SD 148·1]). Figure 2 shows the prevalence of smoking in people presenting with their first episode of psychosis. 34 samples were analysed, from 34 studies (appendix p 3).11, 15, 19, 20, 21, 22, 23, 29, 31, 32, 33, 35, 36, 37, 38, 39, 46, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 In the total sample of smokers, prevalence of smoking in people presenting with their first episode of psychosis was 0·57 (95% CI 0·52–0·62; p<0·0001). Between-sample heterogeneity was significant, with an I2 of 88·0%.
Figure 1.
Search process
Figure 2.
Prevalence of smoking in individuals presenting with their first episode of psychosis
Black diamonds represent prevalences; grey squares represent weights; horizontal lines represent 95% CIs; white diamonds represent subtotal or overall prevalence (dotted line) and 95% CIs. *From random-effects analysis.
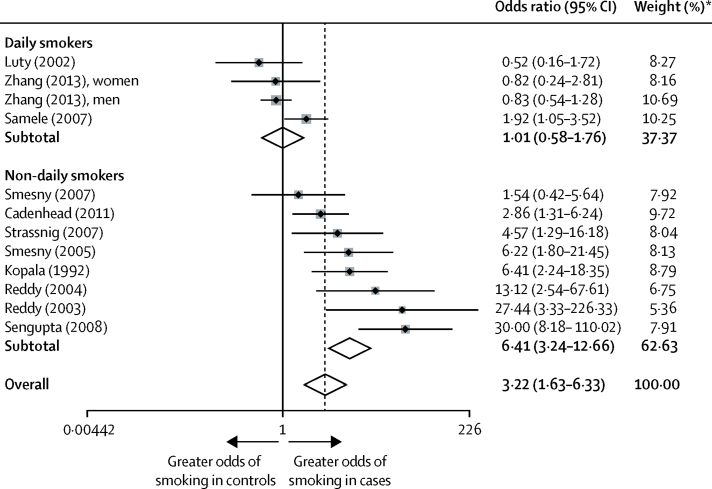
All definitions of smoking (including daily smoking) were included in our analysis of smoking prevalence in people presenting with their first episode of psychosis. 12 samples were obtained for the analysis, from 11 case-control studies.15, 21, 22, 23, 32, 34, 35, 36, 37, 38, 39 Compared with controls, the overall prevalence of smoking in people presenting with their first episode of psychosis was three times higher (odds ratio 3·22, 95% CI 1·63–6·33; p=0·001; figure 3). Between-sample heterogeneity was significant, with an I2 of 82·1%. Findings of Begg's test (p=0·007) and Egger's test (p=0·018) suggested that publication bias might have been present. In the analysis of daily smoking status, four samples were identified, from three studies,15, 21, 32 with an overall odds ratio of 1·01 (95% CI 0·58–1·76; p=0·976; figure 3). Between-sample heterogeneity was significant, with an I2 of 53·3%.
Figure 3.
Odds ratio of smoking daily and non-daily, in people with first-episode psychosis versus controls
Black diamonds represent odds ratios; grey squares represent weights; horizontal lines represent 95% CIs; white diamonds represent subtotal or overall odds ratios (dotted line) and 95% CIs. *From random-effects analysis.
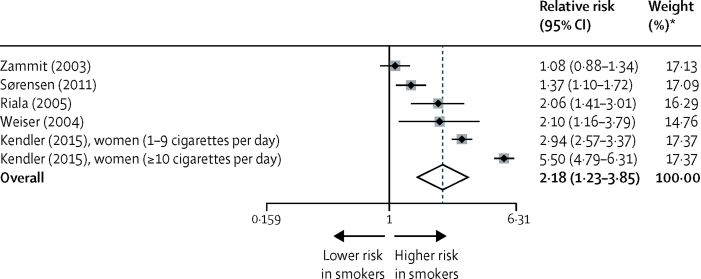
In our analysis of daily tobacco use and risk of psychotic disorder, five longitudinal prospective studies were identified of six samples from the general population (all samples measured risk of schizophrenia).18, 19, 20, 46, 66 Compared with non-smokers, the incidence of new psychotic disorders in daily smokers was higher (overall risk ratio 2·18, 95% CI 1·23–3·85; p=0·007; figure 4). Between-sample heterogeneity was significant, with an I2 of 97·7%. Some evidence was recorded that publication bias contributed to the findings, supported by Egger's test (p=0·002) and Begg's test (p=0·024). We also identified one study comprising a cohort of prodromal individuals,33 in which the risk ratio of developing new-onset psychotic illness in daily smokers versus non-smokers was 7·00 (95% CI 2·35–20·83).
Figure 4.
Risk of psychosis in prospective studies in daily smokers versus non-smokers
Black diamonds represent relative risks; grey squares represent weights; horizontal lines represent 95% CIs; white diamond represents overall relative risk (dotted line) and 95% CI. *From random-effects analysis.
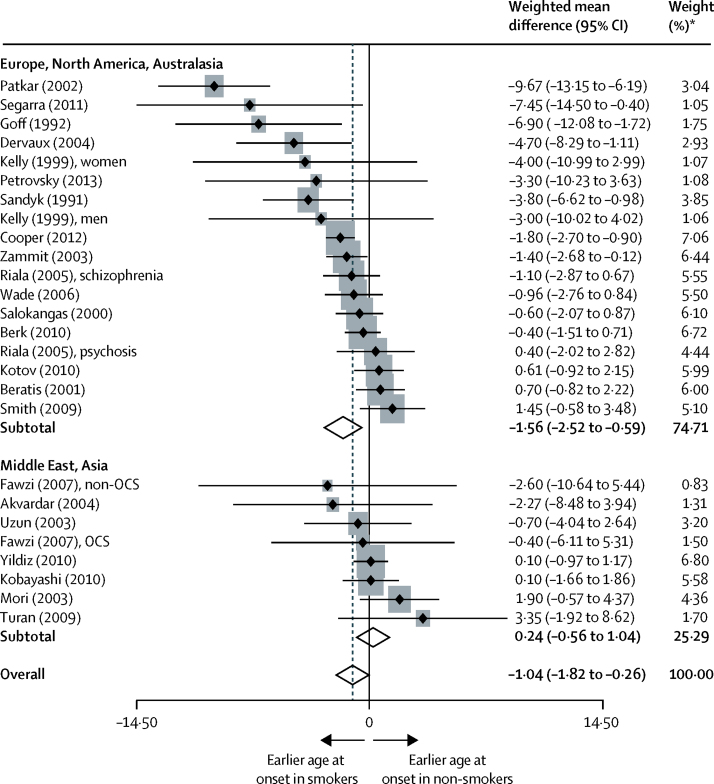
In our analysis of daily tobacco use and age at onset of psychosis compared with non-smokers, 26 samples were included, from 23 studies.13, 19, 25, 26, 27, 28, 30, 40, 41, 42, 43, 46, 53, 59, 61, 67, 68, 69, 70, 71, 72, 73, 74 Daily smokers developed psychotic illness at an earlier age, compared with non-smokers (24·25 years vs 25·63 years; weighted mean difference −1·04, 95% CI −1·82 to −0·26; p=0·009; figure 5). Between-sample heterogeneity was significant, with an I2 of 66·3%. No evidence was recorded that publication bias contributed to the findings, as shown by Egger's test (p=0·149) and Begg's test (p=0·103). When the analysis was divided according to the study country,75 a significant difference was noted between group means (one-way ANOVA F=4·32; p=0·049). Our analysis was based on data showing differences in the age at initiation of smoking in countries in Europe, North America, and Australasia (eg, Australia, Finland, Spain, Sweden, and the USA) versus countries in Asia and the Middle East (eg, Egypt, Japan, and Turkey); specifically, women in Asian countries were seen to begin smoking at a later age. In Europe, North America, and Australasia, 18 samples from 16 studies were analysed,13, 19, 25, 26, 28, 40, 41, 42, 43, 46, 53, 59, 61, 67, 68, 70 with a weighted mean difference of −1·56 (95% CI −2·52 to −0·59; p=0·002; figure 5). In Asia and the Middle East, eight samples from seven studies were analysed,27, 30, 69, 71, 72, 73, 74 with a weighted mean difference of 0·24 (−0·56 to 1·04; p=0·554). No between-sample heterogeneity was recorded, with an I2 of 0%.
Figure 5.
Difference in age at onset of psychosis, in countries around the world, for daily smokers versus non-daily smokers
Black diamonds represent weighted mean differences; grey squares represent weights; horizontal lines represent 95% CIs; white diamonds represent subtotal or overall weighted mean differences (dotted line) and 95% CIs. OCS=obsessive-compulsive symptoms. *From random-effects analysis.
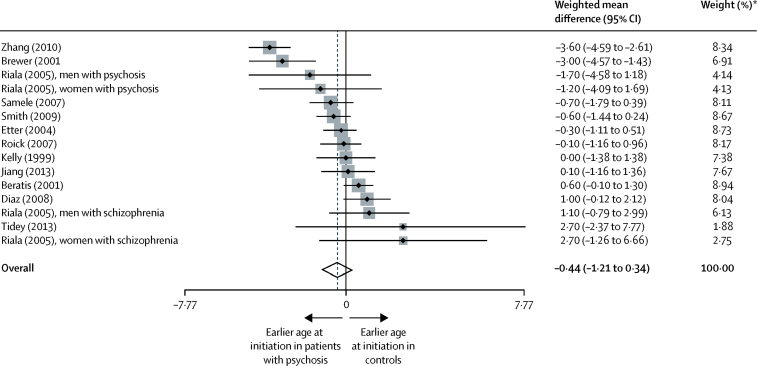
In the analysis of age at initiation of smoking in people with psychosis compared with controls, 15 samples were identified from 12 studies.12, 14, 19, 24, 26, 31, 32, 43, 44, 45, 53, 76 Age at initiation of smoking cigarettes did not differ between patients with psychosis and controls, with a weighted mean difference of −0·44 (95% CI −1·21 to 0·34; p=0·270; figure 6). Between-sample heterogeneity was significant, with an I2 of 81·0%. No evidence was recorded that publication bias contributed to the findings, supported by Egger's test (p=0·957) and Begg's test (p>0·99).
Figure 6.
Difference in age at initiation of smoking in patients with established psychosis versus controls
Black diamonds represent weighted mean differences; grey squares represent weights; horizontal lines represent 95% CIs; white diamond represents overall weighted mean difference (dotted line) and 95% CI. *From random-effects analysis.
Discussion
The findings of our systematic review and meta-analysis show that daily tobacco use is associated with an increased risk of psychotic disorder and an earlier age at onset of psychotic illness. However, the effect of smoking seems to be modest.
The prevalence of smoking in patients presenting with their first episode pf psychosis was 57% (95% CI 52–62). From our analysis of case-control studies, the overall risk of smoking in individuals having their first episode of psychosis was three times higher than that for non-smokers, with a suggestion of possible publication bias. However, when we restricted the analysis to three studies in which daily cigarette smoking was specified, the association disappeared. Our findings differ from those of a previous meta-analysis,9 in which risk of smoking in first-episode psychosis was much higher, but a different set of studies were included in that meta-analysis. Our analysis consisted of studies published up to 2014, and we included two cohorts from China,15 a country with a lower prevalence of smoking than Europe and North America. To acknowledge differences between countries, we analysed cohorts from the general population around the world (appendix p 3).77 Few studies reported daily smoking status.
Analysis of five longitudinal prospective studies18, 19, 20, 46, 66 showed that the risk of psychotic disorder was increased modestly by daily smoking. In two other studies that did not meet our inclusion criteria—one of prodromal psychosis33 and another looking at the risk of developing non-affective psychosis in individuals who had been smoking for at least 9 years78—the risk was much higher.
In our analysis, daily use of tobacco was associated with an earlier onset of psychosis compared with non-smokers. These findings conflict with previous reports,7, 10 in which no relation was noted between smoking status and age at onset of psychosis. This discrepancy could be accounted for by differences in culture—eg, in countries such as the USA and the UK, both boys and girls initiate smoking at an earlier age (around 17 years old),28, 32, 43 whereas in countries such as Egypt and Turkey, boys start to smoke earlier than do girls.79 In a Chinese study,80 the mean age at onset of regular smoking in a cohort with schizophrenia was 20·8 years. Possible reasons for taking up smoking early could be related to self-medication for symptoms of anxiety or isolated psychosis and might be shared with other risk factors for psychosis, such as early life stressors or living in an urban setting. Therefore, for individuals at increased risk, smoking could have an additive effect.
In our analysis, people with psychosis began smoking 0·44 years earlier than did controls, although this difference was not significant. Findings of another meta-analysis estimated a pooled interval of 5·8 years between age at initiation of daily tobacco use and onset of psychosis.9 In another study, children who started smoking at 15 years or younger were more likely to develop non-affective psychosis than were individuals smoking at a later age.78
In view of all our findings, we think that the earlier onset of psychosis and higher risk in smokers of developing psychosis (albeit based on few studies) calls into question the self-medication hypothesis. The excess of smoking nicotine by people with psychosis has been assumed to be secondary to several factors associated with psychosis, subsumed under the umbrella term self-medication. Nicotine use has been suggested to reduce cognitive deficits and symptoms (such as visuospatial working memory and attention deficits,81 working memory, and selective attention),82 P50 inhibition,83 sensory gating, smooth pursuit, and antisaccadic eye movements.84 Nicotine has also been postulated to reduce sedating and other effects of antipsychotic drugs28 and to diminish negative symptoms of psychosis. Use of nicotine at a young age could be attributed to self-medication for anxiety in individuals at the prodromal stage of illness. However, a 2008 review stated that the tobacco industry monitored or directly funded research promoting the self-medication hypothesis, in particular, biological research.85 Furthermore, direct behavioural evidence has failed to show attentional benefits with nicotine by comparison with placebo, in smokers with schizophrenia versus controls.86
With the Bradford Hill criteria, which consider the strength, consistency, specificity, temporality, biological gradient, plausibility, coherence, experiment, and analogy of an association,87 we propose that smoking could have a causal role in psychosis. First, with respect to strength, the association noted in prospective studies undertaken in the general population shows a modest increase in relative risk. Second, the findings seem to be consistent between different populations.7, 10 Third, with respect to temporality, although people with psychosis do not smoke at an earlier age compared with the general population, evidence suggests that an excess of smoking precedes the onset of psychotic illness.9 Fourth, daily smoking seems to have a greater effect on positive symptoms of psychosis.88 Fifth, plausible mechanisms have been proposed between nicotine and the dopamine system, which have been corroborated in epidemiological and laboratory studies. Finally, the effects of nicotine exposure on the dopamine system might have similarities to psychosis.70, 89, 90 The specificity criterion cannot be applied to smoking, because smoking affects a substantial number of disease processes. Also, the experiment criterion is not met because models of important aspects of psychotic illness in animals such as delusions and hallucinations are impossible to achieve.
In implicating nicotine to have a causal role in psychosis (the plausibility part of the Bradford Hill criteria), we must consider whether nicotine has an effect on the dopamine system, since the hypothesis of excess striatal dopamine is the leading pathogenic theory of schizophrenia.91 One of the strongest links between the environment and Parkinson's disease (a dopamine deficiency disorder, in some ways the opposite of schizophrenia) is the inverse relation between nicotine and risk of Parkinson's disease.90 In vivo, nicotine might increase dopamine release directly (measured by PET in the dorsal–ventral striatum and basal ganglia) to a similar degree as other drugs of misuse.70, 91 Another mechanism by which nicotine could cause a change in the dopamine system could be through induction of supersensitivity of D2 receptors, which has been proposed as an explanatory mechanism for several risk factors for schizophrenia and as a common pathway for psychotic symptoms.92 This idea has been corroborated by work in animal models suggesting that nicotine exposure might increase D2 high-affinity receptors.93 Finally, genes coding for nicotine dependence and smoking behaviour—CHRNA3, CHRNA5, and CHRNB4—were identified in the biggest genome-wide association study of schizophrenia to date,94 giving more biological plausibility to this argument.
The main limitation of our study is the small number of longitudinal prospective studies included in the analysis of risk of developing psychosis between smokers and non-smokers. Another substantial limitation is our difficulty in obtaining the exact consumption of substances other than tobacco (eg, cannabis), because very few studies measured or controlled for these variables objectively (appendix pp 1–2). The scant measurement of these potential confounding factors is a clear source of bias.
Future studies, particularly longitudinal and prospective studies with larger sample sizes, should investigate the relation between daily smoking, sporadic smoking, nicotine dependence, and development of psychotic disorders. Adjustment should be made for the effects of other substances of misuse, enabling stringent examination of whether nicotine has a causal role in the development of psychosis. Cigarette smoking might be a hitherto neglected modifiable risk factor for psychosis, but confounding and reverse causality are possible. Notwithstanding, in view of the clear benefits of smoking cessation programmes in this population,95 every effort should be made to implement change in smoking habits in this group of patients.
For the Cochrane handbook see http://handbook.cochrane.org
This online publication has been corrected. The corrected version first appeared at thelancet.com/psychiatry on July 15, 2015
Acknowledgments
Acknowledgments
Our work was funded by the NIHR Maudsley Biomedical Research Centre. We thank Roman Kotov, Stan Zammit, Mark Weiser, Darryl Wade, and John McGrath, who made their data available to us. We also thank Ann McNeill, who commented on a previous draft of our report.
Contributors
PG did the literature search, extracted and selected articles, did the primary analysis, and wrote the report. SJ extracted and selected articles and wrote the report. JHM and RMM formulated the research question and wrote the report.
Declaration of interests
We declare no competing interests.
Supplementary Material
Many patients with psychosis also smoke - could tobacco use be implicated in the condition's onset? Sameer Jauhar and James MacCabe join Hannah Cagney to explore the association.
References
- 1.De Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Eranti SV, MacCabe JH, Bundy H, Murray RM. Gender difference in age at onset of schizophrenia: a meta-analysis. Psychol Med. 2013;43:155–167. doi: 10.1017/S003329171200089X. [DOI] [PubMed] [Google Scholar]
- 4.Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci Biobehav Rev. 2011;35:1779–1787. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Di Forti M, Iyegbe C, Sallis H. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry. 2012;72:811–816. doi: 10.1016/j.biopsych.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 7.Sara G, Burgess P, Malhi GS, Whiteford H, Hall W. Differences in associations between cannabis and stimulant disorders in first admission psychosis. Schizophr Res. 2013;147:216–222. doi: 10.1016/j.schres.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68:555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- 9.Myles N, Newall HD, Curtis J, Nielssen O, Shiers D, Large M. Tobacco use before, at, and after first-episode psychosis: a systematic meta-analysis. J Clin Psychiatry. 2012;73:468–475. doi: 10.4088/JCP.11r07222. [DOI] [PubMed] [Google Scholar]
- 10.Myles N, Newall H, Compton MT, Curtis J, Nielssen O, Large M. The age at onset of psychosis and tobacco use: a systematic meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1243–1250. doi: 10.1007/s00127-011-0431-3. [DOI] [PubMed] [Google Scholar]
- 11.deRuiter WK, Cheng C, Gehrs M, Langley J, Dewa CS. Substance abuse and smoking among a Canadian cohort of first episode psychosis patients. Community Ment Health J. 2013;49:815–821. doi: 10.1007/s10597-013-9634-2. [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, See YM, Subramaniam M, Lee J. Investigation of cigarette smoking among male schizophrenia patients. PLoS One. 2013;8:e71343. doi: 10.1371/journal.pone.0071343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrovsky N, Ettinger U, Quednow BB. Nicotine enhances antisaccade performance in schizophrenia patients and healthy controls. Int J Neuropsychopharmacol. 2013;16:1473–1481. doi: 10.1017/S1461145713000011. [DOI] [PubMed] [Google Scholar]
- 14.Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Ahnallen CG. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2013;15:121–129. doi: 10.1093/ntr/nts098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XY, Chen DC, Xiu MH. Cigarette smoking, psychopathology and cognitive function in first-episode drug-naive patients with schizophrenia: a case-control study. Psychol Med. 2013;43:1651–1660. doi: 10.1017/S0033291712002590. [DOI] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, for the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sørensen HJ, Mortensen EL, Reinisch JM, Mednick SA. A prospective study of smoking in young women and risk of later psychiatric hospitalization. Nord J Psychiatry. 2011;65:3–8. doi: 10.3109/08039481003786386. [DOI] [PubMed] [Google Scholar]
- 19.Riala K, Hakko H, Isohanni M, Pouta A, Räsänen P. Is initiation of smoking associated with the prodromal phase of schizophrenia? J Psychiatry Neurosci. 2005;30:26–32. [PMC free article] [PubMed] [Google Scholar]
- 20.Weiser M, Reichenberg A, Grotto I. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am J Psychiatry. 2004;161:1219–1223. doi: 10.1176/appi.ajp.161.7.1219. [DOI] [PubMed] [Google Scholar]
- 21.Luty J, Kelly C, McCreadie RG. Smoking habits, body mass index and risk of heart disease: prospective 2½-year follow-up of first episode schizophrenic patients. J Subst Use. 2002;7:15–18. [Google Scholar]
- 22.Kopala LC, Clark C, Hurwitz T. Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr Res. 1992;8:245–250. doi: 10.1016/0920-9964(93)90022-b. [DOI] [PubMed] [Google Scholar]
- 23.Smesny S, Kinder D, Willhardt I. Increased calcium-independent phospholipase A2 activity in first but not in multiepisode chronic schizophrenia. Biol Psychiatry. 2005;57:399–405. doi: 10.1016/j.biopsych.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Diaz FJ, Velásquez DM, Susce MT, de Leon J. The association between schizophrenia and smoking: unexplained by either the illness or the prodromal period. Schizophr Res. 2008;104:214–219. doi: 10.1016/j.schres.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Sandyk R, Kay SR. Tobacco addiction as a marker of age at onset of schizophrenia. Int J Neurosci. 1991;57:259–262. doi: 10.3109/00207459109150699. [DOI] [PubMed] [Google Scholar]
- 26.Beratis S, Katrivanou A, Gourzis P. Factors affecting smoking in schizophrenia. Compr Psychiatry. 2001;42:393–402. doi: 10.1053/comp.2001.26273. [DOI] [PubMed] [Google Scholar]
- 27.Fawzi MH, Fawzi MM, Khedr HH, Fawzi MM. Tobacco smoking in Egyptian schizophrenia patients with and without obsessive-compulsive symptoms. Schizophr Res. 2007;95:236–246. doi: 10.1016/j.schres.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Goff DC, Henderson DC, Amico E. Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry. 1992;149:1189–1194. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- 29.Zabala A, Eguiluz JI, Segarra R. Cognitive performance and cigarette smoking in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci. 2009;259:65–71. doi: 10.1007/s00406-008-0835-6. [DOI] [PubMed] [Google Scholar]
- 30.Uzun O, Cansever A, Basoğlu C, Ozşahin A. Smoking and substance abuse in outpatients with schizophrenia: a 2-year follow-up study in Turkey. Drug Alcohol Depend. 2003;70:187–192. doi: 10.1016/s0376-8716(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 31.Brewer WJ, Pantelis C, Anderson V. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158:107–115. doi: 10.1176/appi.ajp.158.1.107. [DOI] [PubMed] [Google Scholar]
- 32.Samele C, Patel M, Boydell J, Leese M, Wessely S, Murray R. Physical illness and lifestyle risk factors in people with their first presentation of psychosis. Soc Psychiatry Psychiatr Epidemiol. 2007;42:117–124. doi: 10.1007/s00127-006-0135-2. [DOI] [PubMed] [Google Scholar]
- 33.Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res. 2007;151:151–154. doi: 10.1016/j.psychres.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cadenhead KS. Startle reactivity and prepulse inhibition in prodromal and early psychosis: effects of age, antipsychotics, tobacco and cannabis in a vulnerable population. Psychiatry Res. 2011;188:208–216. doi: 10.1016/j.psychres.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy R, Keshavan M, Yao JK. Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr Res. 2003;62:205–212. doi: 10.1016/s0920-9964(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 36.Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull. 2004;30:901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- 37.Sengupta S, Parrilla-Escobar MA, Klink R. Are metabolic indices different between drug-naïve first-episode psychosis patients and healthy controls? Schizophr Res. 2008;102:329–336. doi: 10.1016/j.schres.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Smesny S, Klemm S, Stockebrand M. Endophenotype properties of niacin sensitivity as marker of impaired prostaglandin signalling in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2007;77:79–85. doi: 10.1016/j.plefa.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Strassnig M, Miewald J, Keshavan M, Ganguli R. Weight gain in newly diagnosed first-episode psychosis patients and healthy comparisons: one-year analysis. Schizophr Res. 2007;93:90–98. doi: 10.1016/j.schres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper J, Mancuso SG, Borland R, Slade T, Galletly C, Castle D. Tobacco smoking among people living with a psychotic illness: the second Australian Survey of Psychosis. Aust N Z J Psychiatry. 2012;46:851–863. doi: 10.1177/0004867412449876. [DOI] [PubMed] [Google Scholar]
- 41.Dervaux A, Baylé FJ, Laqueille X. Nicotine use in schizophrenia and disinhibition. Psychiatry Res. 2004;128:229–234. doi: 10.1016/j.psychres.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Patkar AA, Gopalakrishnan R, Lundy A, Leone FT, Certa KM, Weinstein SP. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190:604–610. doi: 10.1097/00005053-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Kelly C, McCreadie RG. Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry. 1999;156:1751–1757. doi: 10.1176/ajp.156.11.1751. [DOI] [PubMed] [Google Scholar]
- 44.Etter M, Mohr S, Garin C, Etter JF. Stages of change in smokers with schizophrenia or schizoaffective disorder and in the general population. Schizophr Bull. 2004;30:459–468. doi: 10.1093/oxfordjournals.schbul.a007092. [DOI] [PubMed] [Google Scholar]
- 45.Roick C, Fritz-Wieacker A, Matschinger H. Health habits of patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2007;42:268–276. doi: 10.1007/s00127-007-0164-5. [DOI] [PubMed] [Google Scholar]
- 46.Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingsson T, Lewis G. Investigating the association between cigarette smoking and schizophrenia in a cohort study. Am J Psychiatry. 2003;160:2216–2221. doi: 10.1176/appi.ajp.160.12.2216. [DOI] [PubMed] [Google Scholar]
- 47.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 48.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baeza I, Graell M, Moreno D. Cannabis use in children and adolescents with first episode psychosis: influence on psychopathology and short-term outcome (CAFEPS study) Schizophr Res. 2009;113:129–137. doi: 10.1016/j.schres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Compton MT, Kelley ME, Ramsay CE. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 2009;166:1251–1257. doi: 10.1176/appi.ajp.2009.09030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hakko H, Lintunen J, Lappalainen J, Mäkikyrö T, Räsänen P, Timonen M, the STUDY-70 Workgroup Nicotine use and dependence and their association to psychiatric disorders in a large sample of adolescent psychiatric inpatients. Addict Behav. 2006;31:1873–1880. doi: 10.1016/j.addbeh.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Ilomäki R, Riala K, Hakko H, the Study 70 Workgroup Temporal association of onset of daily smoking with adolescent substance use and psychiatric morbidity. Eur Psychiatry. 2008;23:85–91. doi: 10.1016/j.eurpsy.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Smith GN, Wong H, MacEwan GW. Predictors of starting to smoke cigarettes in patients with first episode psychosis. Schizophr Res. 2009;108:258–264. doi: 10.1016/j.schres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 54.Perez-Iglesias R, Mata I, Pelayo-Teran JM. Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naïve population. Schizophr Res. 2009;107:115–121. doi: 10.1016/j.schres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 55.Smith GN, Macewan GW, Kopala LC. Prenatal tobacco exposure in first-episode psychosis. Schizophr Res. 2010;119:271–272. doi: 10.1016/j.schres.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Harrison I, Joyce EM, Mutsatsa SH. Naturalistic follow-up of co-morbid substance use in schizophrenia: the West London first-episode study. Psychol Med. 2008;38:79–88. doi: 10.1017/S0033291707000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curtis J, Henry C, Watkins A, Newall H, Samaras K, Ward PB. Metabolic abnormalities in an early psychosis service: a retrospective, naturalistic cross-sectional study. Early Interv Psychiatry. 2011;5:108–114. doi: 10.1111/j.1751-7893.2011.00262.x. [DOI] [PubMed] [Google Scholar]
- 58.Hides L, Cotton SM, Berger G. The reliability and validity of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in first-episode psychosis. Addict Behav. 2009;34:821–825. doi: 10.1016/j.addbeh.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Kotov R, Guey LT, Bromet EJ, Schwartz JE. Smoking in schizophrenia: diagnostic specificity, symptom correlates, and illness severity. Schizophr Bull. 2010;36:173–181. doi: 10.1093/schbul/sbn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrigón ML, Gurpegui M, Ruiz-Veguilla M. Temporal relationship of first-episode non-affective psychosis with cannabis use: a clinical verification of an epidemiological hypothesis. J Psychiatr Res. 2010;44:413–420. doi: 10.1016/j.jpsychires.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Berk M, Henry LP, Elkins KS. The impact of smoking on clinical outcomes after first episode psychosis: longer-term outcome findings from the EPPIC 800 follow-up study. J Dual Diagn. 2010;6:212–234. [Google Scholar]
- 62.McCreadie RG, Paterson JR, Blacklock C, the Scottish Schizophrenia Research Group Smoking habits and plasma lipid peroxide and vitamin E levels in never-treated first-episode patients with schizophrenia. Br J Psychiatry. 2000;176:290–293. doi: 10.1192/bjp.176.3.290. [DOI] [PubMed] [Google Scholar]
- 63.McEvoy JP, Brown S. Smoking in first-episode patients with schizophrenia. Am J Psychiatry. 1999;156:1120–1121. doi: 10.1176/ajp.156.7.1120a. [DOI] [PubMed] [Google Scholar]
- 64.Wade D, Harrigan S, Edwards J, Burgess PM, Whelan G, McGorry PD. Patterns and predictors of substance use disorders and daily tobacco use in first-episode psychosis. Aust N Z J Psychiatry. 2005;39:892–898. doi: 10.1080/j.1440-1614.2005.01699.x. [DOI] [PubMed] [Google Scholar]
- 65.Hilti CC, Delko T, Orosz AT. Sustained attention and planning deficits but intact attentional set-shifting in neuroleptic-naïve first-episode schizophrenia patients. Neuropsychobiology. 2010;61:79–86. doi: 10.1159/000265133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kendler KS, Lönn SL, Sundquist J, Sundquist K. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.15010126. published online June 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Segarra R, Zabala A, Eguíluz JI. Cognitive performance and smoking in first-episode psychosis: the self-medication hypothesis. Eur Arch Psychiatry Clin Neurosci. 2011;261:241–250. doi: 10.1007/s00406-010-0146-6. [DOI] [PubMed] [Google Scholar]
- 68.Wade D, Harrigan S, Edwards J, Burgess PM, Whelan G, McGorry PD. Course of substance misuse and daily tobacco use in first-episode psychosis. Schizophr Res. 2006;81:145–150. doi: 10.1016/j.schres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Akvardar Y, Tumuklu M, Akdede BB, Ulas H, Kitis A, Alptekin K. Substance use among patients with schizophrenia in a university hospital. Bull Clin Psychopharmacol. 2004;14:191–197. [Google Scholar]
- 70.Salokangas RK, Vilkman H, Ilonen T. High levels of dopamine activity in the basal ganglia of cigarette smokers. Am J Psychiatry. 2000;157:632–634. doi: 10.1176/appi.ajp.157.4.632. [DOI] [PubMed] [Google Scholar]
- 71.Yıldız M, Yazıcı A, Böke O. Demographic and clinical characteristics in schizophrenia: a multi center cross-sectional case record study. Turk Psikiyatri Derg. 2010;21:213–224. (in Turkish). [PubMed] [Google Scholar]
- 72.Kobayashi M, Ito H, Okumura Y, Mayahara K, Matsumoto Y, Hirakawa J. Hospital readmission in first-time admitted patients with schizophrenia: smoking patients had higher hospital readmission rate than non-smoking patients. Int J Psychiatry Med. 2010;40:247–257. doi: 10.2190/PM.40.3.b. [DOI] [PubMed] [Google Scholar]
- 73.Mori T, Sasaki T, Iwanami A. Smoking habits in Japanese patients with schizophrenia. Psychiatry Res. 2003;120:207–209. doi: 10.1016/s0165-1781(03)00191-4. [DOI] [PubMed] [Google Scholar]
- 74.Turan T, Dolu N, Ozsoy S, Esel E. Effects of smoking on P50 waveform in schizophrenic patients. Bull Clin Psychopharmacol. 2009;19:227–235. [Google Scholar]
- 75.Gelman A. Analysis of variance? Why it is more important than ever. Ann Stat. 2005;33:1–53. [Google Scholar]
- 76.Zhang XY, Li CB, Li M. Smoking initiation and schizophrenia: a replication study in a Chinese Han population. Schizophr Res. 2010;119:110–114. doi: 10.1016/j.schres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Ng M, Freeman MK, Fleming TD. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 78.McGrath JJ, Najman J, Williams G, Bor W, Scott J. Age at first tobacco use and risk of subsequent psychosis-related outcomes: a birth cohort and nested sibling-pair study. In: Abstracts of the 14th International Congress on Schizophrenia Research. Schizophr Bull. 2013;39(suppl 1):S1–376. (S69). [Google Scholar]
- 79.Giovino GA, Mirza SA, Samet JM, the GATS Collaborative Group Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–679. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 80.Hou YZ, Xiang YT, Yan F. Cigarette smoking in community-dwelling patients with schizophrenia in China. J Psychiatr Res. 2011;45:1551–1556. doi: 10.1016/j.jpsychires.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 81.Sacco KA, Termine A, Seyal A. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- 82.Jacobsen LK, D'Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 83.Chen XS, Li CB, Smith RC, Xiao ZP, Wang JJ. Differential sensory gating functions between smokers and non-smokers among drug-naive first episode schizophrenic patients. Psychiatry Res. 2011;188:327–333. doi: 10.1016/j.psychres.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 84.Schnur P, Hoffman AC. Nicotinic modulation of attentional deficits in schizophrenia. In: Lubow RE, Weiner I, editors. Latent inhibition: cognition, neuroscience and applications to schizophrenia. Cambridge University Press; New York: 2010. pp. 477–499. [Google Scholar]
- 85.Prochaska JJ, Hall SM, Bero LA. Tobacco use among individuals with schizophrenia: what role has the tobacco industry played? Schizophr Bull. 2008;34:555–567. doi: 10.1093/schbul/sbm117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol Psychiatry. 2013;74:436–443. doi: 10.1016/j.biopsych.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bradford Hill A. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 88.Krishnadas R, Jauhar S, Telfer S, Shivashankar S, McCreadie RG. Nicotine dependence and illness severity in schizophrenia. Br J Psychiatry. 2012;201:306–312. doi: 10.1192/bjp.bp.111.107953. [DOI] [PubMed] [Google Scholar]
- 89.Seeman P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther. 2011;17:118–132. doi: 10.1111/j.1755-5949.2010.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quik M. Smoking, nicotine and Parkinson's disease. Trends Neurosci. 2004;27:561–568. doi: 10.1016/j.tins.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 91.Brody AL, Olmstead RE, London ED. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- 92.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Novak G, Seeman P, Le Foll B. Exposure to nicotine produces an increase in dopamine D2(high) receptors: a possible mechanism for dopamine hypersensitivity. Int J Neurosci. 2010;120:691–697. doi: 10.3109/00207454.2010.513462. [DOI] [PubMed] [Google Scholar]
- 94.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2 doi: 10.1002/14651858.CD007253.pub3. CD007253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Many patients with psychosis also smoke - could tobacco use be implicated in the condition's onset? Sameer Jauhar and James MacCabe join Hannah Cagney to explore the association.