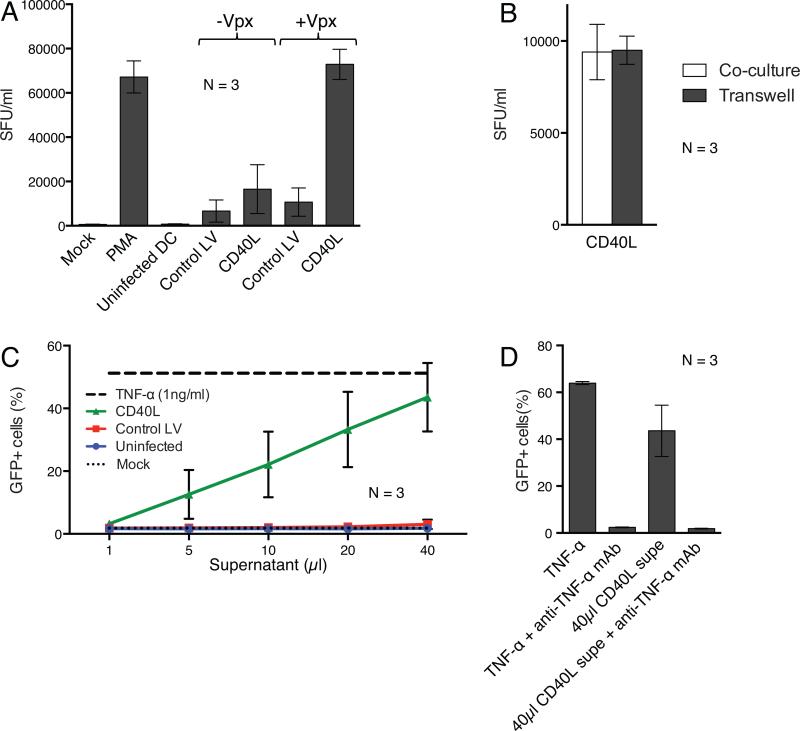
Figure 7. DCs transduced with CD40L lentiviral expression vectors reactivate latent HIV-1 provirus expression.
(a) DCs from three donors were mock infected or transduced with lentiviral vectors containing or lacking Vpx. 24 hours later, the cells were cocultured with infected ACH-2 cells and after two days, infectious virus in the culture supernatant was quantified on TZM-bl reporter cells as the number of spot forming units per milliliter (SFU)/ml. As controls, ACH-2 cells were cultured alone (Mock) or treated with PMA. The data are the average of three donors with error bars indicating mean +/− SD. (b) DCs transduced with Vpx-containing lentiviral vectors expressing CD40L were cocultured with ACH-2 cells or in separate chambers of a transwell plate. The data are the average of three donors with error bars indicating mean +/− SD. (c) DCs from three donors were mock infected or transduced with Vpx-containing lentiviral vectors. After 72 hours, serial dilutions of culture supernatant were assayed by adding increasing amounts of supernatant to J-Lat cells. Control J-Lat cells were untreated or treated with TNF-α. 24 hours later, the number of GFP+ cells was quantified by flow cytometry. The data are the average of three donors with error bars indicating mean +/− SD. (d) Similar to (c), except that TNF-α supernatant from CD40L transduced DCs was pretreated with an anti-TNF-α mAb prior to incubation with the J-Lat cells.