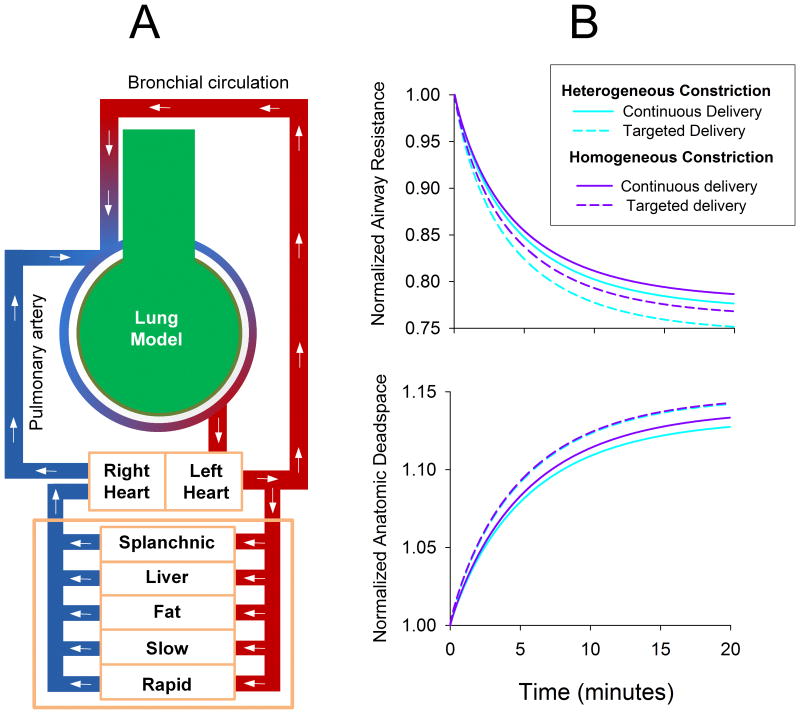
Figure 3.
(A) Computational model used to simulate the administration of volatile anesthetic during mechanical ventilation of a canine lung. Lung model consisted of a 10 generation airway tree of cylindrical tubes, terminating in homogeneous tissues elastances and gas volumes. Canine-specific lengths and diameters [65] were assigned and normalized to achieve an anatomic dead space of 150 mL. Airways were constricted either 1) homogeneously by reducing all diameters by 30%, or 2) heterogeneously by stochastically reducing all diameters by 25 to 35%. Following bronchoconstriction, inhaled anesthetic was deposited directly onto airway luminal surfaces to elicit dose-dependent ASM relaxation. Bronchial blood flow also allowed the anesthetic to reach poorly-ventilated regions, prior to emptying into the pulmonary circulation, which perfused the terminal compartments uniformly. Systemic blood flow was simulated via a 5-compartment model to predict local anesthetic concentrations in various organs over time [61]. Simulated ventilation was performed using a tidal volume of 350 mL, with constant inspiratory flow and passive exhalation at 20 min-1 for 20 minutes. Inspired air contained equal doses of isoflurane either: 1) continuously throughout inspiration; or 2) only during the last portion of inspiration with a volume equal to the anatomic dead space. Flow calculations, particle transport and deposition, and receptor binding kinetics were all simulated using the model of Amin, et al. [60]. (B) Normalized lung resistance and anatomic deadspace for homogeneous and heterogeneous model conditions for administration of isoflurane in continuous or targeted delivery modes.