Introduction
Volumetric laser endomicroscopy (VLE) is second-generation optical coherence tomography (OCT) that is being used for real-time, high-resolution cross-sectional imaging in Barrett’s esophagus [Suter et al. 2008; Yun et al. 2006]. This technology captures images up to 3 mm below the mucosa at a 7 µm resolution in real time. The VLE platform (NvisionVLE™ Imaging System, NinePoint Medical, Bedford, MA, USA) provides real-time, high-resolution cross-sectional imaging using a balloon catheter with scanning optics. This technology has been shown to be sensitive in the detection of dysplasia in Barrett’s esophagus [Evans et al. 2006]. While this technology is being used in the USA in the esophagus, there have been no studies or cases evaluating it in other areas of the gastrointestinal tract. The balloon platform of VLE allows cross-sectional imaging of the gastrointestinal tract if the balloon can collapse the lumen of the area being imaged to allow for adequate scanning of the gastrointestinal tract layers. At our institution, we use VLE routinely for the management of Barrett’s esophagus. We present a case where VLE was used in the rectum in order to provide high-resolution images to help define the anatomy of a neoplastic-appearing polyp prior to surgery. Informed consent has been obtained from the patient discussed in this case report for use of their anonymized details.
Case
A 66-year-old man with ulcerative colitis was referred by the surgical service for endosonographic evaluation of a rectal polyp with high-grade dysplasia seen on a recent colonoscopy. The colonoscopy diagnosing the polyp showed no other lesions. We were asked to rule out invasive disease prior to surgery as this information would help determine the surgical plan. The surgical plan was to remove the polyp by surgical full-thickness resection.
On flexible sigmoidoscopy there was a 4 cm, sessile, slightly depressed polyp (Paris classification 0–IIc) located in the rectum at the border of the dentate line (Figure1a). The slight depression was concerning for invasive disease. Narrow-band imaging showed a type IV Kudo classification mucosal pit pattern with irregular dilated vessels concerning for a neoplastic process (Figure 1b) [Kudo et al. 1996; Tischendorf et al. 2007]. Endoscopic ultrasound (EUS) evaluation with a radial array echoendoscope (GF-UE160-AL5, Olympus America, Center Valley, PA, USA) at 10 MHz frequency showed intact gastrointestinal layers in the region of the polyp without lymphadenopathy (Figure 2). The submucosa and muscularis propria were intact. No focal lesion was identified. The EUS finding was unexpected, as we expected a focal mucosal hypoechoic area or focal thickening in the region of the polyp.
Figure 1.
Rectal sessile polyp on flexible sigmoidoscopy on (a) high-definition endoscopy and (b) narrow-band imaging high-definition endoscopy.
Figure 2.

Endoscopic ultrasound image of the rectum involving the polyp.
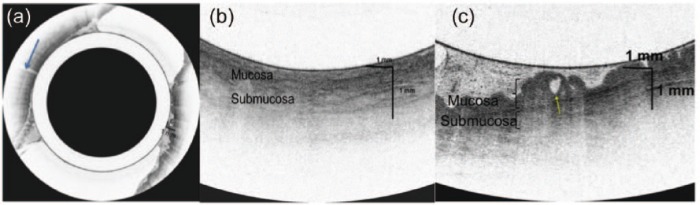
Given that a discrete lesion was not identified and the findings were unexpected, it was unclear whether the lesion was identified on EUS. Therefore VLE was performed as this technology delivers up to a 25 times higher spatial resolution than EUS. A 20 mm balloon catheter that contained the VLE optical probe was used through the channel of a gastroscope to obtain a cross- sectional scan at the area of the polyp (Figure 3). The scan showed a normal uniform rectal mucosa opposite the wall of the polyp (Figure 4a and b). At the site of the polyp there was an irregular epithelial surface and an atypical gland in the mucosa at the site of the polyp with a normal submucosa below it (Figure 4c). The balloon contains a registration line that can be visualized endoscopically and on the VLE scan (Figure 4a). Matching up these lines allows for endoscopic localization of abnormalities. VLE helped to confirm that the polyp was limited to the mucosa, as a focal atypical area was seen at the site of the polyp, with a normal submucosa below this area. This high-resolution imaging delineated the anatomy and thus allowed for a defined surgical plan. Given the findings on VLE, the surgeon altered his management. Instead of performing a full-thickness resection, he resected the polyp endoscopically using the endoscopic mucosal resection (EMR) technique. Pathology confirmed negative margins. The final pathology was consistent with a mucosal polyp with high-grade dysplasia (Figure 5). There was submucosa present in the pathology specimen that was free of polyp. The final histology correlated well to the presurgery imaging studies.
Figure 3.

Volumetric laser endomicroscopy being performed with a 20 mm balloon containing the optical probe.
Figure 4.
(a) Circumferential volumetric laser endomicroscopy (VLE) images of the rectum that contained the polyp. The registration line (blue arrow) helps to localize the polyp when compared to the registration line on the endoscopic view. (b) Magnified view of the normal rectal mucosa with a uniform epithelial surface and normal layers. (c) Cross-sectional image of VLE at the polyp site showing an irregular epithelial surface and atypical gland (yellow arrow) in the mucosa with intact layers below it.
Figure 5.
(a) Surgical histology of the rectal polyp showing the dysplasia is limited to the mucosa. (b) Magnified view of the mucosa showing high-grade dysplasia.
Discussion
Although VLE has been designed for use in the esophagus, this case shows that VLE can be successfully performed in the rectum. It can be complementary to EUS as shown in this case. There may be an advantage for VLE in superficial mucosal lesions given the higher spatial resolution. As seen in this case, the EUS resolution was not high enough to visualize the polyp, which is a limitation of EUS. It is important to note that VLE provides high-resolution images of the mucosa and submucosa; it may not always image the deeper layers like endoscopic ultrasound [Sivak et al. 2000].
In this case, the patient was specifically referred for advanced imaging of the rectal polyp to determine if a resection should take place. In centers where advanced imaging is not available, it is reasonable to perform a staging EMR. This can be both diagnostic and therapeutic. Although this is likely more cost effective, it is more invasive and subjects a patient to possible adverse events of EMR. In patients with lesions that invade the submucosa and require surgery, advanced imaging can spare an EMR.
To our knowledge, this is the first reported case of the use of VLE in imaging a rectal polyp. Therefore further studies are needed prior to recommending widespread use of VLE in the rectum. Previous reports have looked at OCT in the rectum and lower gastrointestinal tract in defining the corresponding histologic layers [Sivak et al. 2000; Consolo et al. 2008; Westphal et al. 2005].
The location of the rectal polyp in this case, near the dentate line, was optimal for imaging. The smaller diameter lumen in this area of the rectum allowed for contact of the mucosa on the balloon and thus provided high-quality images of the mucosa that contained the polyp. However a full circumferential view with the lateral rectal walls could not be obtained due to the larger diameter rectum. In this case the lateral walls did not need to be imaged as the polyp clearly was localized to one wall. The registration line showed that we imaged the wall that contained the polyp. It is possible that if this lesion were more proximal in the rectum imaging would not have been possible with the 20 mm balloon, the largest balloon available with the VLE platform. Future studies using VLE in the rectum may have to use larger balloons containing the optical probe, especially if full circumferential views are desired.
To date, the majority of studies looking at OCT and VLE in the gastrointestinal tract focus on the esophagus. Mucosal-based dysplastic lesions in the esophagus that are precursors to adenocarcinoma have atypical glands and abnormal surface maturation [Evans et al. 2006]. It can be extrapolated from the OCT esophagus data that dysplastic lesions in the rectum have similar features. In this case report the rectal polyp had an atypical epithelial gland present.
Overall, VLE is a promising new technology that is gaining widespread acceptance for esophageal imaging in the USA. With further studies, this technology can be applied to other areas of the gastrointestinal tract to provide high-resolution imaging. As demonstrated here, high-resolution imaging can visualize abnormal lesions that help guide management decisions.
Acknowledgments
Authors contributions are as follows: conception and design (AJT and DVS); analysis and interpretation of the data (AJT, DVS); drafting of the article (AJT, ASV, KS, CF, and DVS); critical revision of the article for important intellectual content (AJT, DVS, ASV, KS, and CF); final approval of the article (AJT, DVS, ASV, CF, and KS).
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Arvind J. Trindade, Division of Gastroenterology, Hepatology, and Nutrition, North Shore University Hospital, Hofstra North Shore-LIJ School of Medicine, 300 Community Drive, Levitt Building, 4th Floor, Manhasset, NY 11030, USA.
Keith Sultan, Division of Gastroenterology, Hepatology, and Nutrition, North Shore University Hospital, Hofstra North Shore-LIJ School of Medicine, Manhasset, NY, USA.
Arunan S. Vamadevan, Division of Gastroenterology, Hepatology, and Nutrition, North Shore University Hospital, Hofstra North Shore-LIJ School of Medicine, Manhasset, NY, USA
Cathy Fan, Department of Pathology, Hofstra North Shore-LIJ School of Medicine, Manhasset, NY, USA.
Divyesh V. Sejpal, Division of Gastroenterology, Hepatology, and Nutrition, North Shore University Hospital, Hofstra North Shore-LIJ School of Medicine, Manhasset, NY, USA
References
- Consolo P., Strangio G., Luigiano C., Giacobbe G., Pallio S., Familiari L. (2008) Optical coherence tomography in inflammatory bowel disease: prospective evaluation of 35 patients. Dis Col Rectum 51: 1374–1380. [DOI] [PubMed] [Google Scholar]
- Evans J., Poneros J., Bouma B., Bressner J., Halpern E., Shishkov M., et al. (2006) Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol 4: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo S., Tamura S., Nakajima T., Yamano H., Kusaka H., Watanabe H. (1996) Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 44: 8–14. [DOI] [PubMed] [Google Scholar]
- Sivak M., Kobayashi K., Izatt J., Rollins A., Ung-Runyawee R., Chak A., et al. (2000) High-resolution endoscopic imaging of the GI tract using optical coherence tomography. Gastrointest Endosc 51: 474–479. [DOI] [PubMed] [Google Scholar]
- Suter M., Vakoc B., Yachimski P., Shishkov M., Lauwers G., Mino-Kenudson M., et al. (2008) Comprehensive microscopy of the esophagus in human patients with optical frequency domain imaging. Gastrointest Endosc 68: 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischendorf J., Wasmuth H., Koch A., Hecker H., Trautwein C., Winograd R. (2007) Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy 39: 1092–1096. [DOI] [PubMed] [Google Scholar]
- Westphal V., Rollins A., Willis J., Sivak M., Izatt J. (2005) Correlation of endoscopic optical coherence tomography with histology in the lower-GI tract. Gastrointest Endosc 61: 537–546. [DOI] [PubMed] [Google Scholar]
- Yun S., Tearney G., Vakoc B., Shishkov M., Oh W., Desjardins A., et al. (2006) Comprehensive volumetric optical microscopy in vivo. Nat Med 12: 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]