Fig. 2. Nox-mediated regulation of the cytoskeleton.
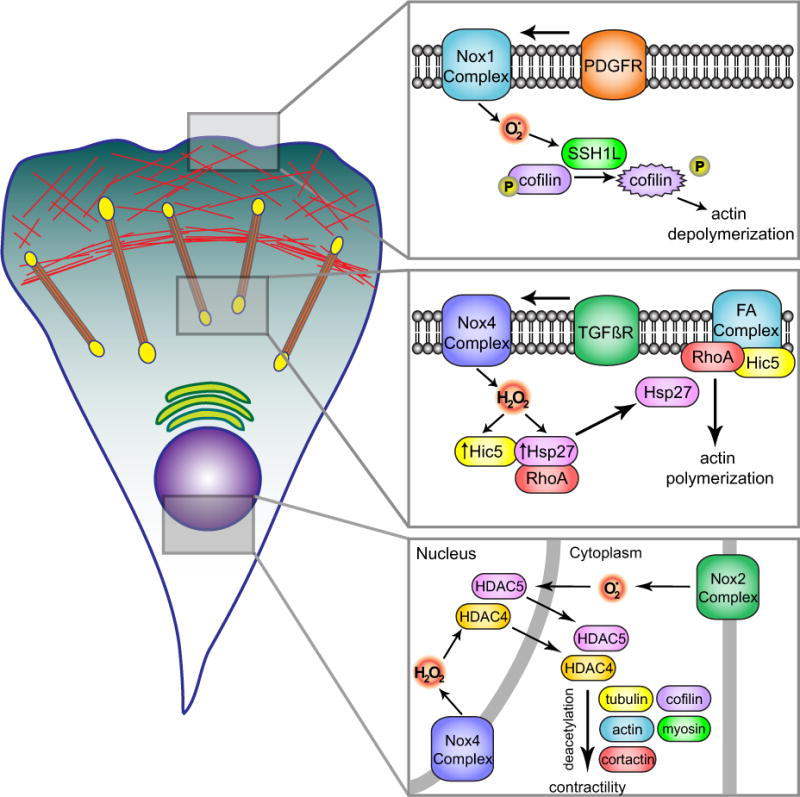
Different cytoskeletal pathways are directly influenced by Nox-produced ROS. Top insert: PDGF-induced actin polymerization uses Nox1-derived superoxide that after being dismutased to H2O2, activates SSH1L phosphatase by oxidation of inhibitory 14-3-3 proteins. SSH1L phosphatase induces dephosphorylation and activation of the actin binding protein cofilin stimulating actin depolymerization. Middle insert: Participation of Nox4 in TGFβ-induced FA formation. After TGFßR activation, Nox4 produces H2O2, which mediates the expression of Hic-5 and the chaperone protein Hsp27. Hic-5 localizes to FA and mediates TGFβ induced adhesive forces while Hsp27 aids Hic-5 localization to FA and RhoA activation-induced actin polymerization. Bottom insert: Nox complexes participate in the redox-depending shuttling of HDACs between nucleus and cytosol. Nox2 and Nox4 oxidize HDACs leading to their translocation to the cytosol and the consequent deacetylation of cytoskeleton-related proteins which leads to changes in cellular contractility.
