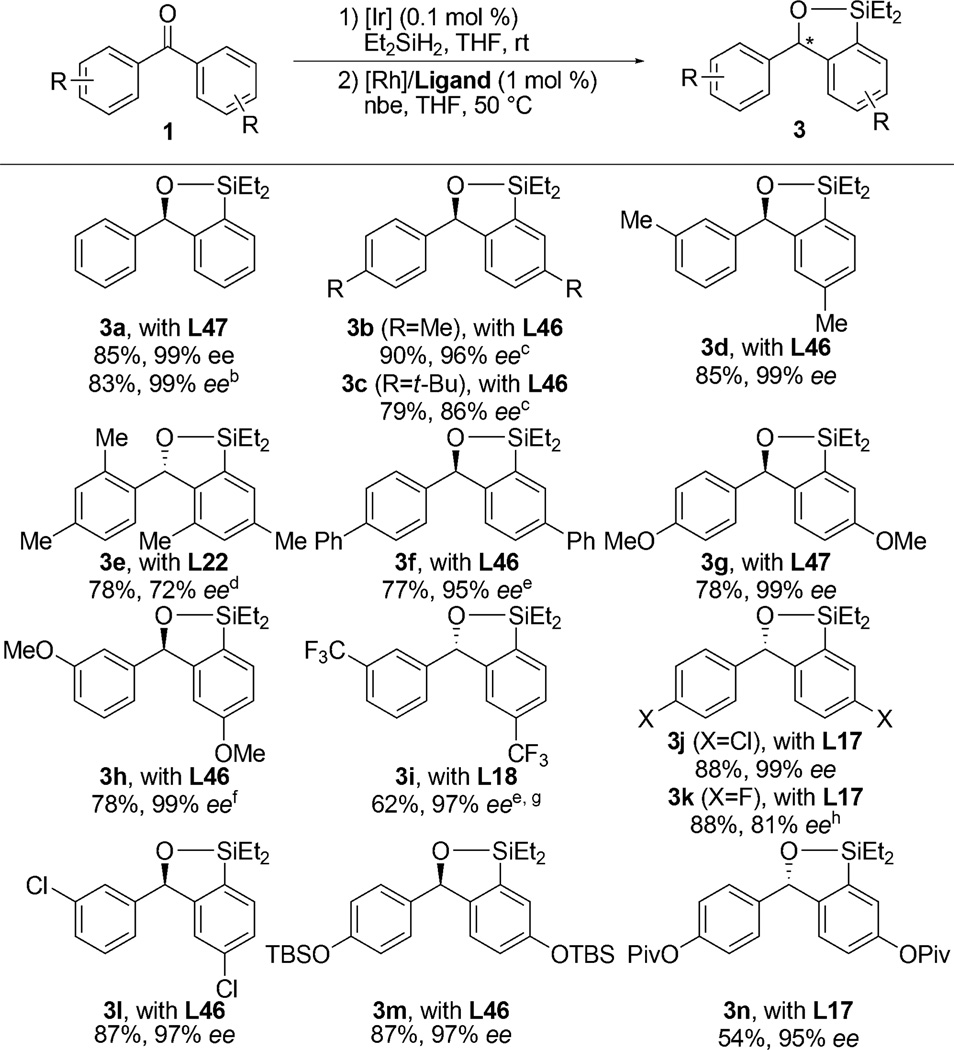
Table 1.
Scope of Enantioselective C–H Silylationa
Isolated yields for reactions conducted on a 1.0 mmol scale following purification by silica gel chromatography. The ee values were determined by chiral HPLC analysis. The absolute configuration was assigned by analogy (see SI for details).
5.0 mmol scale.
The ee values were determined after iodination.
The reaction was conducted at 80 °C.
0.1 mol % [Ir(cod)OMe]2 was used for the first step.
The corresponding diarylmethanol was used as a substrate.
The ee value was determined after Tamao–Fleming oxidation.
The reaction was conducted at room temperature.