Figure 3.
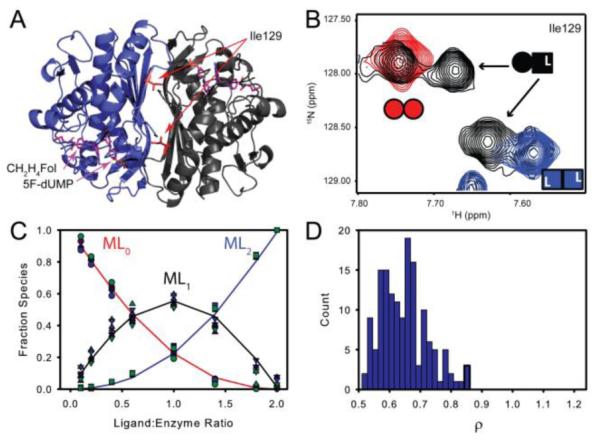
Both TS active sites have similar affinity for the 5F-dUMP-CH2H4Fol “di-ligand”. A&B) In NMR spectra, at intermediate titration points (black, (B)), resonances from a subset of residues near the dimer interface (e.g. Ile 129 in (A)) have chemical shifts from the singly bound state that are different from the free (red, Panel (B)) and doubly bound states (blue (B)). For these residues there are four resonances total at intermediate titration points as the singly bound state produces two peaks: one from the free subunit and one from the bound subunit (B). C) Global fit of peak intensities from the four resonances having all three states resolved in NMR spectra. Circles and squares represent free and doubly bound data, respectively. Upward triangles are from the free subunit of singly bound species and downward tringles are from the bound subunit. D) Histogram of ρ (K2/K1 ratio) from fits of 150 Monte Carlo simulated datasets.
