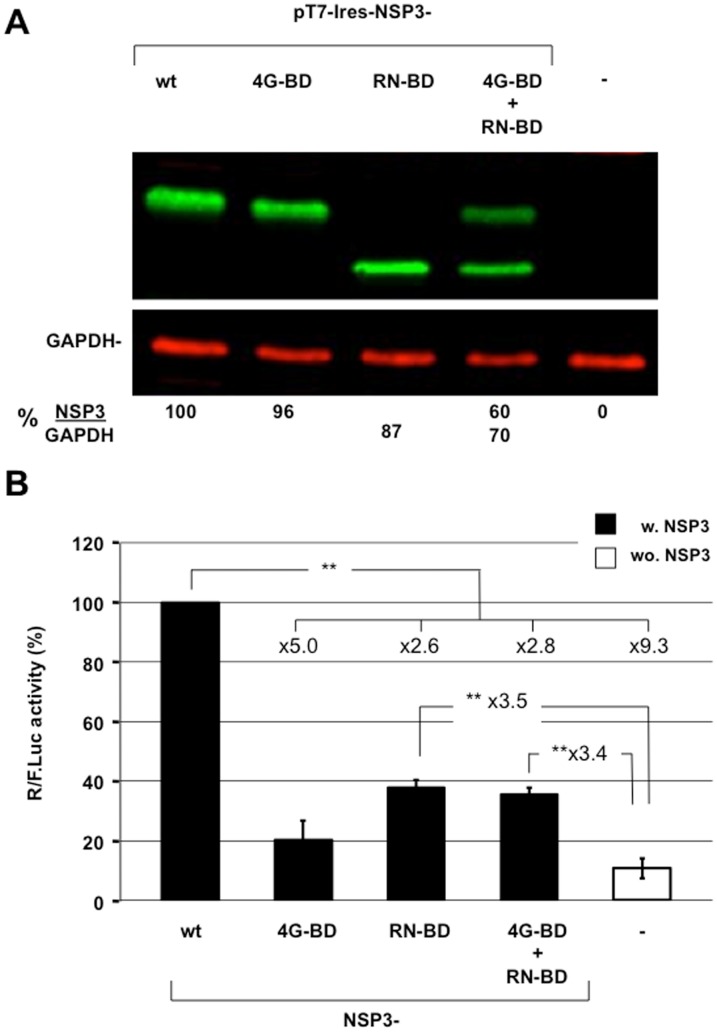
Fig 4. The importance of the NSP3 eIF4G- and RNA-binding domains.
A: Expression of wild type or mutated NSP3. Lysate from BSRT7 cells transfected for 24 h with the expression vectors encoding wild type NSP3 (wt); NSP3 mutated at the RNA-binding domain (4G-BD) or the eIF4G-binding domain (RN-BD) alone or in combination; or no NSP3 (NSP3-KO) was analyzed using western blotting with an anti-NSP3 rabbit polyclonal antibody and a mouse monoclonal antibody against the cellular protein GAPDH (used as a loading control). The ratio of NSP3 versus GAPDH fluorescence (NSP3/GAPDH) is indicated at the bottom of the figure. B: BSRT7 cells expressing wild type or mutated NSP3 were electroporated with the capped R-RNA and standard RNA. The Renilla and firefly luciferase activities were measured 6 hours after electroporation. The Renilla to firefly luciferase ratio is relative to the control (R-RNA in cells expressing NSP3-RF), which was considered 100. The data are the mean ± SEM of three independent experiments in triplicate. The fold increases are indicated and a two-tailed Student’s t-test was used (*p<0.05; **p<0.01).