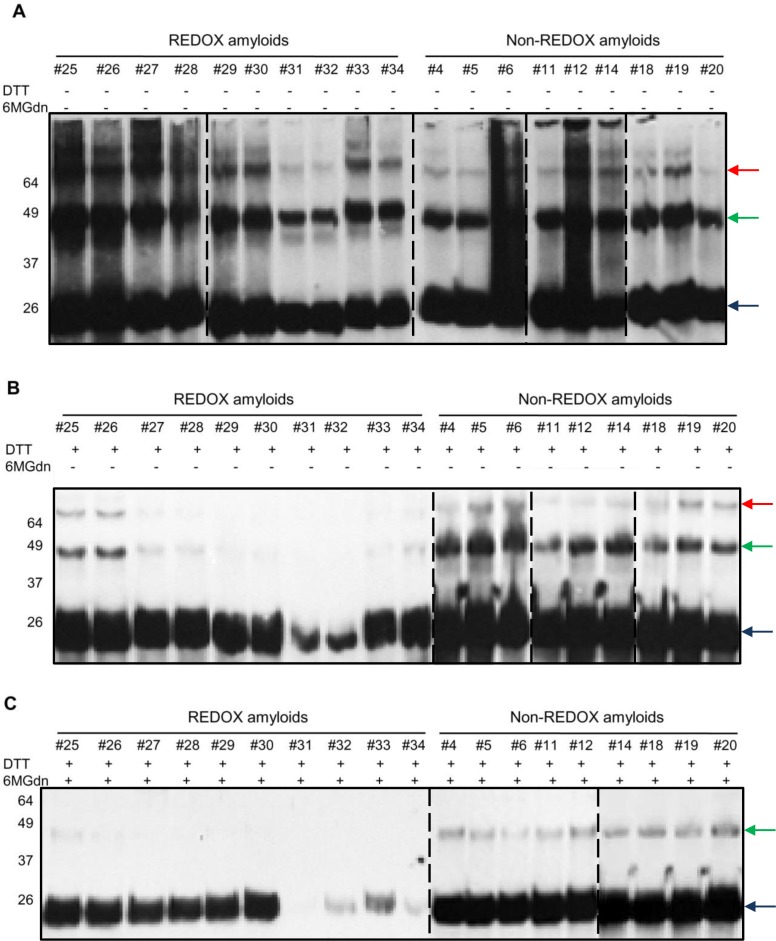
Fig 3. Western blot analysis of amyloid fibrils performed under reducing and non-reducing SDS-PAGE.
Monomeric (indicated by blue arrow) recMoPrP(23–231) was converted into amyloid forms by intermolecular disulfide linkage following the REDOX process. Western blotting of non-reducing Sodium Dodecyl Sulphate—PolyAcrylamide Gel Electrophoresis (SDS-PAGE) showing the conversion of recMoPrP(23–231) to amyloids is indicated by dimer (green arrow), trimer (red arrow), and more complex structures in both processes (A). Western blotting of reducing SDS-PAGE after treatment of amyloid with Dithiothreitol reducing agent (DTT) shows the decrease in signals of dimer, trimer and more complicated structures in all lanes of amyloid samples from REDOX-process (B). Western blotting of reducing SDS-PAGE of amyloid after a 3-day treatment with denaturant (6M Gdn-HCl), and subsequently with reducing agent DTT shows only monomeric recMoPrP(23–231) bands and the disappearance of more complicated structures in all lanes of amyloid samples in REDOX process (C). Western blots were performed using Fab D18 monoclonal antibody (1μg/mL). Blots were developed with the enhanced chemiluminescent system (ECL, Amersham Biosciences) and visualized on Hyperfilm (Amersham Biosciences)