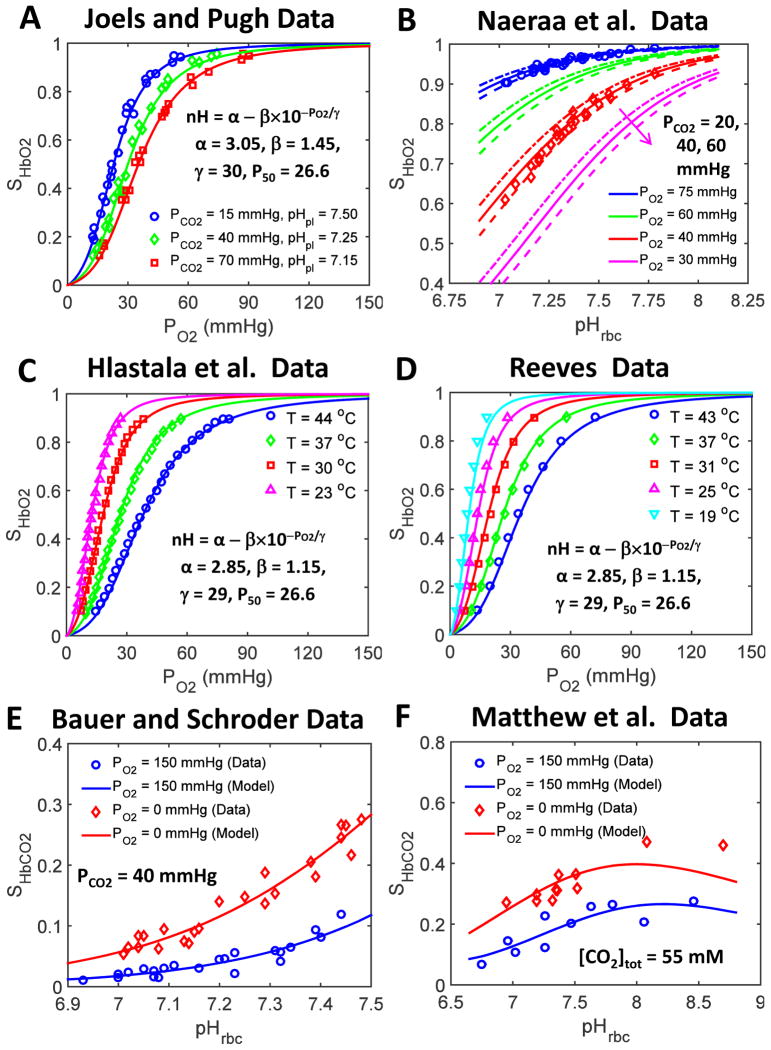
Figure 3. Comparison of our improved SHbO2 and SHbCO2 model simulations to the diverse experimental data available in the literature under non-standard physiological conditions.
(A) HbO2 dissociation curves obtained from our improved SHbO2 model (i.e. modified SHbO2 model with variable cooperativity hypothesis for O2-Hb binding (variable nH)) compared with the data of Joels and Pugh (1958) obtained at various pHpl and PCO2 levels with T = 37 °C. The inset shows the expression for the variable nH and the governing parameter values that produce the best fit of the model to these data sets. In addition, these model fittings characterize the pH and PCO2 dependencies of P50 in Eqs. 9a and 9b. (B) SHbO2 levels obtained from our improved SHbO2 model compared to the data of Naeraa et al. (1963) obtained as a function of pHrbc for different PO2 and PCO2 levels at 37 °C. Other details are as for panel A. (C,D) HbO2 dissociation curves obtained from our improved SHbO2 model compared with the data of Hlastala et al. (1977) and Reeves (1980) obtained for various values of T with pHpl and PCO2 fixed at 7.4 and 40 mmHg, respectively. The insets show the expression for the variable nH and the governing parameter values that enable best fits of the model to these data sets. In addition, these model fittings characterize the temperature dependency of P50 in Eq. 9d. (E,F) SHbCO2 levels obtained from our improved SHbCO2 model compared with the data of Bauer and Schröder (1972) and Matthew et al. (1977) obtained as a function of pHrbc in the oxygenated (high PO2) and deoxygenated (zero PO2) blood with PCO2 fixed at 40 mmHg (T = 37 °C) in the former experiments and total [CO2] fixed at 55 mM (T = 30 °C) in the latter experiments. These model fittings provide the estimates of the equilibrium constants associated with the binding of CO2 to oxygenated and deoxygenated Hb as well as the ionization constants of oxygenated and deoxygenated Hb.