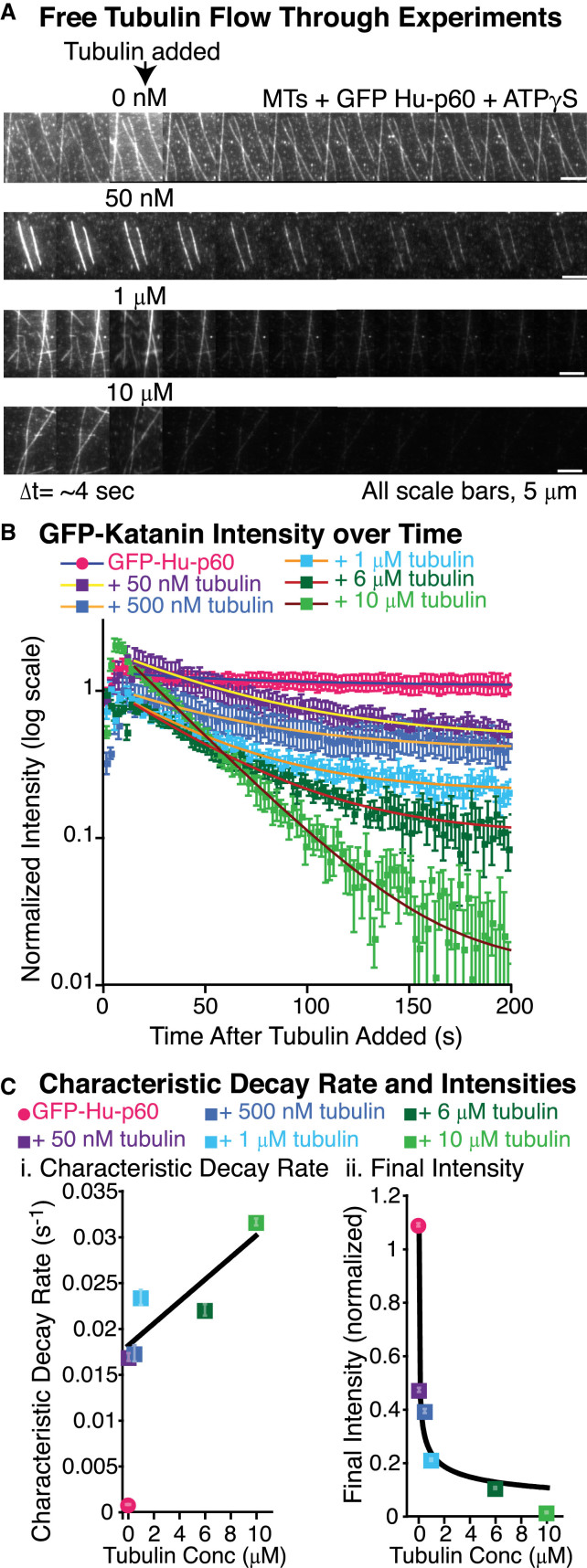
Figure 4.
Katanin has a higher affinity for free tubulin than microtubules. (A) Example time series of unbinding of GFP-Hu-p60 via free tubulin at concentrations of 0 nM, 50 nM, 1 μM, and 10 μM free tubulin. The data were taken by imaging continuously for several minutes. The time between frames in the time series is 4 s. GFP-Hu-p60 was pre-bound to microtubules with ATPγS prior to starting imaging, allowing the enzyme to bind, but not sever, microtubules. The tubulin was added as indicated in the third frame of the time series. The scale bars are 5 μm. (B) Quantification of the average GFP fluorescence remaining on the microtubules over time after free tubulin was added to the assay. Error bars represent the mean ± SE. The data show a significant decrease in fluorescence on the microtubules when tubulin is added. Each data set was fit to an exponential decay curve. (C) Plots of (i) characteristic decay rates and (ii) final intensities for each condition in (B). (i) Katanin unbinding rates increased in the presence of increasing tubulin concentrations. Error bars represent uncertainty in fit paramters and many are smaller than the symbol. Data fit to Eq. 11 (black line). (ii) Final intensities proportional to the final concentration of katanin bound to the microtubule were fit to a modified Hill Eq. 10 (black curve). For all data displayed, the number of microtubules analyzed was: 0 nM (pink circles, N = 20), 50 nM (purple squares, N = 23), 500 nM (dark blue squares, N = 22), 1 μM (light blue squares, N = 25), 6 μM (dark green squares, N = 25), and 10 μM free tubulin (light green squares, N = 11). All fit equations, parameters, and goodness of fits are given in Table S4.