Abstract
Human induced ecosystem alterations and climate change are expected to drive several species to extinction. In this context, the attention of public opinion, and hence conservationists' efforts, are often targeted towards species having emotional, recreational and/or economical value. This tendency may result in a high number of extinctions happening unnoticed. Among these, many could involve parasites. Several studies have highlighted various reasons why we should care about this, that go far beyond the fact that parasites are amazingly diverse. A growing corpus of evidence suggests that parasites contribute much to ecosystems both in terms of biomass and services, and the seemingly paradoxical idea that a healthy ecosystem is one rich in parasites is becoming key to the whole concept of parasite conservation. Although various articles have covered different aspects of host–parasite co-extinctions, I feel that some important conceptual issues still need to be formally addressed. In this review, I will attempt at clarifying some of them, with the aim of providing researchers with a unifying conceptual framework that could help them designing future studies. In doing this, I will try to draw a more clear distinction between the (co-)evolutionary and the ecological dimensions of co-extinction studies, since the ongoing processes that are putting parasites at risk now operate at a scale that is extremely different from the one that has shaped host–parasite networks throughout million years of co-evolution. Moreover, I will emphasize how the complexity of direct and indirect effects of parasites on ecosystems makes it much challenging to identify the mechanisms possibly leading to co-extinction events, and to predict how such events will affect ecosystems in the long run.
Keywords: Biological invasions, Climate change, Ecological networks, Enemy release, Host-specificity, Host range, Indirect competition, IUCN
Graphical abstract
Highlights
-
•
Parasites play fundamental roles in ecosystems in terms of biomass and services.
-
•
Global biodiversity loss could drive many parasites to extinction.
-
•
The dynamics of host–parasite co-extinctions need further investigation.
-
•
Evolution and ecology should be clearly distinguished in co-extinction studies.
-
•
Modeling the indirect effects of parasite loss on ecosystems could be unfeasible.
1. Introduction
Evidence suggests the sixth mass extinction on the history of the planet is now taking place (Wake and Vredenburg, 2008). Although this can be partly attributed to climate change (Thomas et al., 2004), humans are playing a fundamental role in this process (Kerr and Currie, 1995), by causing habitat loss, by prosecuting target species, and by introducing alien competitors, pests and/or predators (Owens and Bennett, 2000, Clavero and Garcia-Berthou, 2005).
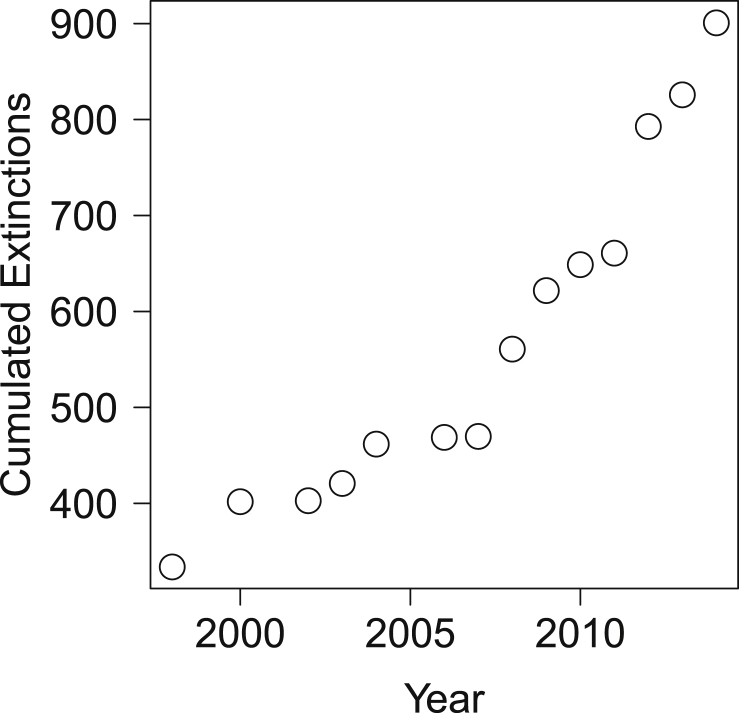
To date, there have been almost one thousand documented extinctions according to IUCN (2014) (Fig.1). Yet, the loss of species that are difficult to be monitored could remain undetected. For example, although thousands of insect extinctions are estimated to have occurred, only 70 have been documented in modern times (Dunn, 2005). Considering that monitoring parasites can be even harder than monitoring insects (and free-living species in general), it is very likely that we are neglecting many parasite extinctions. Nevertheless, only few historical or contemporary co-extinction events have been actually recorded (Dunn et al., 2009). Diamond (1989) argued that the major causes of extinction can be summarized into four categories, which he compared to the four horsemen of the apocalypse. Among these, three (namely, habitat loss, overkill and species invasion) have been extensively investigated. Conversely, the mechanisms and the relevance of the fourth cause, i.e. extinction cascades and/or co-extinctions, have been often underrated, even if their importance has been known for a long time (Stork and Lyal, 1993), and they have been identified by different models as fundamental drivers of species loss (Koh et al., 2004a, Dunn, 2009, Dunn et al., 2009).
Fig. 1.
Cumulative number of documented species extinctions according to IUCN (2014).
This subject, however, has recently received increasing attention, and has been thoroughly investigated in a series of studies focusing on the potential role of co-extinctions in biodiversity loss (see, for example, Koh et al., 2004b, Eklöf and Ebenman, 2006, Borrvall and Ebenman, 2006, Roopnarine, 2006, Rezende et al., 2007, Nichols et al., 2009, Moir et al., 2010, Sahasrabudhe and Motter, 2011).
When studying co-extinctions, it is fundamental to make a clear distinction between mutualistic and host–parasite networks. In principle, the loss of a pollinator can negatively affect a plant species, possibly resulting in a detrimental effect towards other pollinator species using that plant, and hence on the other plants used by these pollinators, and so on. Conversely, the disappearance of a parasite from a host is not expected to have similar effects; on the contrary, one may expect that the disappearance of a parasite would produce a positive effect for its hosts, and hence for other parasite species using those hosts, possibly buffering the effect of the first extinction. However, the system is likely more complicated than this. The next paragraphs will aim at clarifying some of the mechanisms possibly at play.
2. Equal rights for parasites
The loss of parasite species, intuitively, should not represent a threat (and could even provide benefit) to associated hosts. Why, then, should we care about parasite extinctions?
Actually, this question is not new, dating back to the early nineties, when Windsor first emphasized the fact that conservation biologists were much worried about saving free-living species, but not at all about preserving parasites (Windsor, 1990). Since this first claim, Windsor put much effort in trying to spread his idea (Windsor, 1995, Windsor, 1997a, Windsor, 1997b). In his subsequent work, when emphasizing why we should care, he made it clear that preserving parasites is important because their species richness is extraordinary, possibly exceeding that of free-living species (Windsor, 1998).
However, Windsor's plea has been largely ignored for almost twenty years, perhaps because the intrinsic value of biodiversity, as in the case of insects (Dunn, 2005), cannot justify conservation measures unless paired to emotional or recreational value (Kellert, 1997). Recently, the situation has begun to change, and there are scholars proposing active parasite conservation strategies such as establishing parasite refugia (Stringer and Linklater, 2014, Jørgensen, 2015). Yet, the reasons for such proposals are much more complex than a simple quest for preserving all extant species.
Several studies have highlighted how parasites, besides diversity and biomass, provide ecosystems with fundamental functions and services, occupying key positions in food webs (Hudson et al., 2006, Lafferty et al., 2006, Lafferty et al., 2008, Kuris et al., 2008). Therefore, the loss of parasites can trigger a series of long-term, indirect effects whose dynamics and extents are, at the moment, largely unexplored. All this considered, Windsor's claim (1990) may not only be well justified, but could even be an underestimation of the current situation, where parasites, in some cases, should be object of primary attention, being them possible indicators of ecosystem health (Marcogliese and Cone, 1997, Marcogliese, 2005, Whiteman and Parker, 2005).
3. Parasite diversity
The provocative question posed by Costello et al. (2013) – Can we name Earth's species before they go extinct? – summarizes well the idea that having a good knowledge of the starting diversity is the fundamental premise to quantify extinction processes. This problem is far from trivial for free-living species, let alone for parasites.
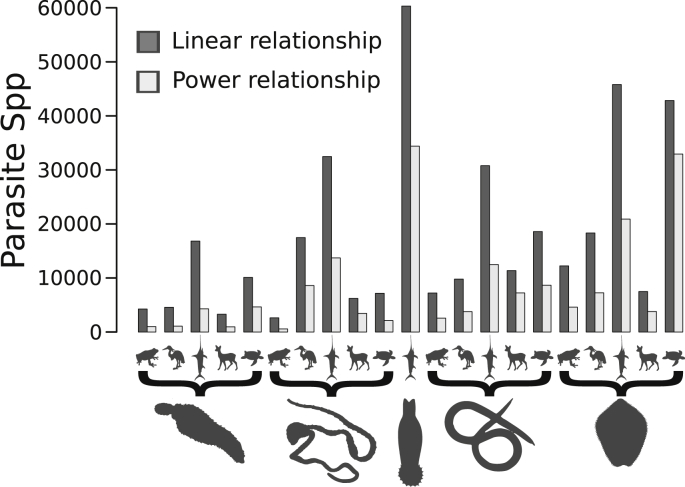
There is still much debate about how many parasites are there (Windsor, 1998, Poulin and Morand, 2000, Dobson et al., 2008, Poulin, 2014, Strona and Fattorini, 2014a). A classical approach to estimate global parasite diversity consists in using a simple linear relationship, and computing the total number of parasite species as Sh × (Pn/Hr), where Pn is the average number of parasite species per host species, Hr is the average host range of a parasite, and Sh is the total number of available host species (Poulin and Morand, 2004). Yet, this approach may severely over-estimate global parasite diversity, due to the fact that the discovery of new parasite species becomes less likely as more host species are investigated, which may lead to non-negligible upwards biases in Pn (Strona and Fattorini, 2014a). A possible alternative approach is that of resampling existing datasets in order to take into account this aspect, by modeling empirically the relationship between the number of examined host species and the number of retrieved parasite species. Using this procedure, Strona and Fattorini (2014a) provided more conservative estimates of global diversity of helminth parasites of vertebrates, which suggest that we are likely overrating global parasite richness (Fig. 2). Yet, limitations in our current knowledge of parasite diversity (Strona and Lafferty, 2013) may affect the reliability of host-specificity and parasite richness per host, possibly biasing estimates of parasite diversity independently from the computational approach (Poulin, 2014). Moreover, thanks to the growing diffusion and technical advances in molecular analysis, the discovery of cryptic species has become more common (Dobson et al., 2008). This could suggest that we are actually under-estimating parasite diversity by counting multiple species on different hosts as one. Yet, most parasitological studies aim at finding new species. As a result, only a minor proportion of host species has been examined at various localities and in several moments, so that we may be under-estimating host ranges of some parasite species (Strona and Fattorini, 2014b). As a coarse approximation, we may assume that these two potential biases can partly compensate one another.
Fig. 2.
Comparison between helminth parasite diversity (for Acantocephala, Cestoda, Monogenea, Nematoda and Trematoda) in vertebrates (amphibians, birds, fish, mammals and reptiles) estimated using, respectively, the approach by Poulin and Morand (2004) (dark grey) and the more recent approach proposed by Strona and Fattorini (2014a) (light grey). Data were obtained from Table 1 in Strona and Fattorini, 2014a, Strona and Fattorini, 2014b, Strona and Fattorini, 2014c).
These uncertainties, besides affecting negatively the accuracy in our knowledge of parasite diversity, may complicate co-extinction studies. A notable example is that of two lices (Campanulotes defectus Tendeiro, 1969 and Columbicola extinctus Malcomson, 1937) that were erroneously thought to have gone co-extinct together with their host, the passenger pigeon Ectopistes migratorius (Linnaeus, 1766). The first lice was shown by Clayton and Price (1999) to be a junior synonym of Campanulotes flavus (Rudow, 1869), that is perfectly alive and parasitizing bronzewings such as Phaps chalcoptera (Latham, 1790) and P. elegans (Temminck, 1809). The second lice was “brought back” from extinction by Price et al. (2000), who showed it to be conspecific with a lice from the extant band-tailed pigeon, Patagioenas fasciata (Say, 1822).
Similar situations could suggest that co-extinction events in parasites are indeed uncommon. Moreover, although host switch events are difficult to document in the wild, laboratory experiments and molecular studies suggest that they could be more common than thought (Perlman and Jaenike, 2003). Besides being possibly key to evolutionary expansion (e.g. Kearn, 1994) and geographical spread (e.g. Mu et al., 2005), host switch could provide parasites with an added mechanism to escape co-extinction, as it has been observed in human and cattle schistosome species (Huyse et al., 2009).
4. Host parasite co-evolution and missing co-extinctions
The number of documented co-extinction events is surprisingly low, especially considering that they could be the main drivers of diversity loss (Dunn et al., 2009). A common assumption in the study of co-extinctions is that specialized species are the most endangered (Dunn et al., 2009, Lafferty, 2012). A paradigmatic example is that of the stomach bot fly Gyrostigma rhinocerontis Hope, 1840, that is a host specific parasite of the critically endangered black rhino Diceros bicornis (Linnaeus, 1758) (IUCN), and that is hence at high risk of co-extinction (Stringer and Linklater, 2014).
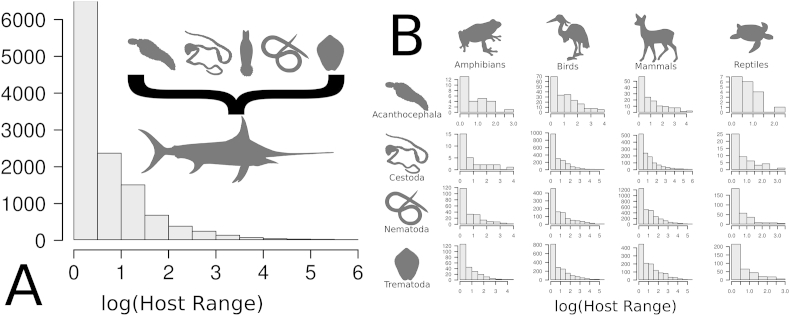
However, the assumption that host range is the main determinant of parasite extinction risk contrasts with the fact that most parasites on earth are highly specialized (Fig. 3; Vázquez et al., 2005). The difficulties in reconciling the commonness of specialization and the relatively small number of observed parasite co-extinctions suggest that, contrary to intuition, the evolution of host specialization does not necessarily increase parasite extinction risk.
Fig. 3.
Distribution of parasite specificity expressed as the logarithm of host range size in fish (A) and terrestrial vertebrates (B). Data for fish parasites (Acantocephala, Cestoda, Monogenea, Nematoda and Trematoda) were collected from FishPest (Strona and Lafferty, 2012). Data for parasites of terrestrial vertebrates (Acantocephala, Cestoda, Nematoda and Trematoda for amphibians, birds, mammals and reptiles) were collected from the Natural Museum History database (http://www.nhm.ac.uk). Since (as to June 11th 2015) all amphibians in the database are erroneously classified as reptiles, information was corrected using Catalogue of Life (http://www.catalogueoflife.org/). Y-axes indicate parasite species numbers.
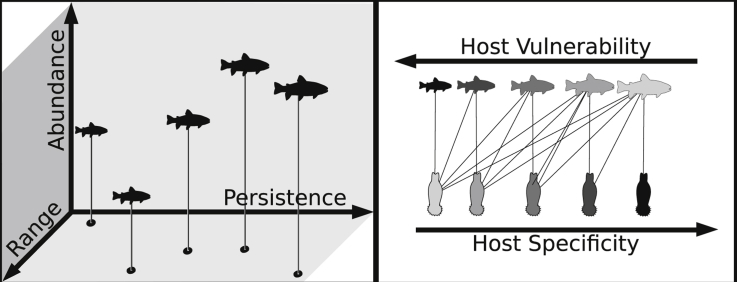
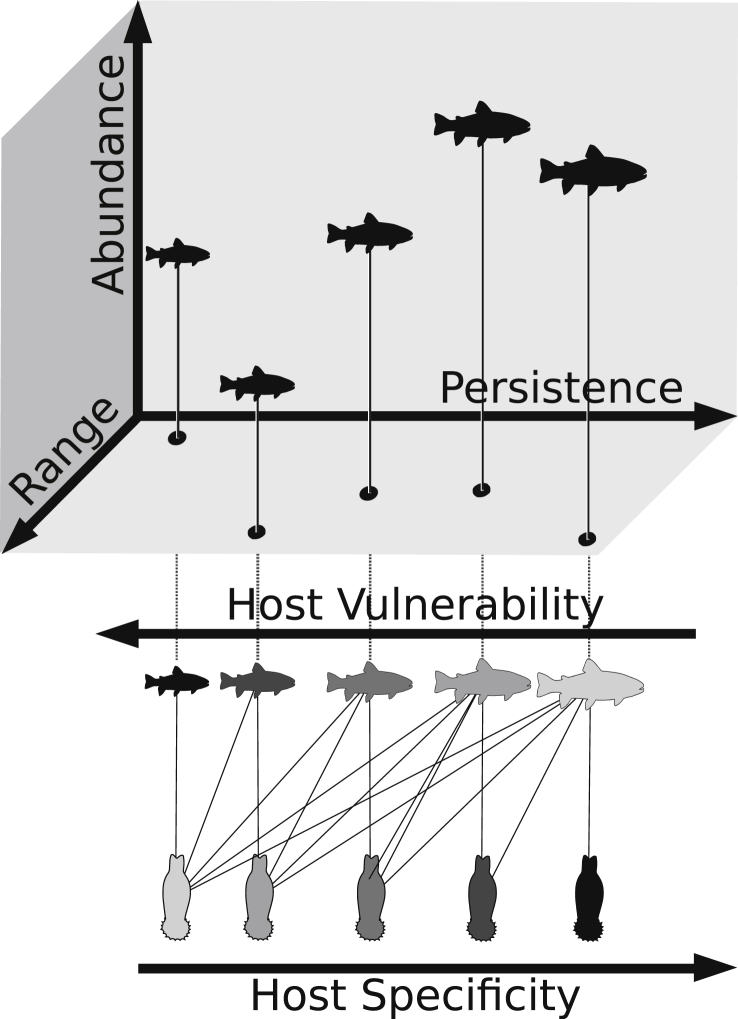
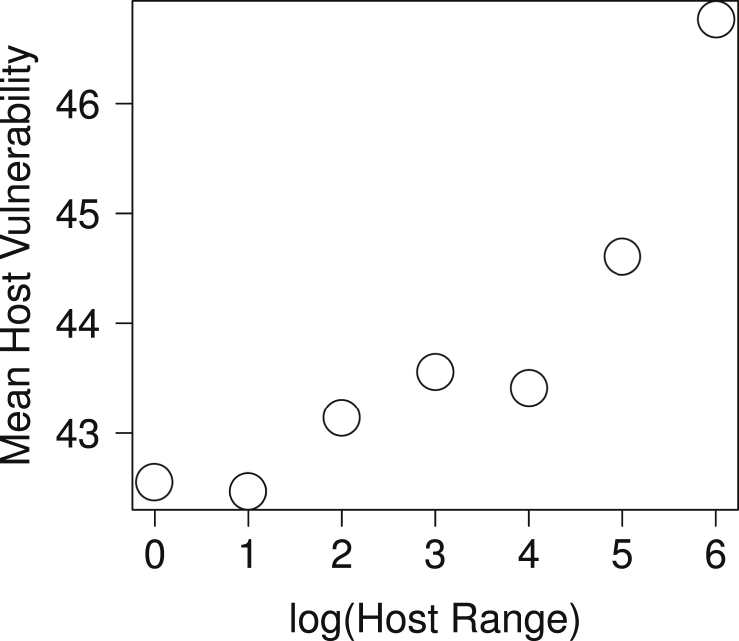
Comparing patterns of parasite specificity and host vulnerability in fish-parasite networks, Strona et al. (2013) provided a simple explanation to this puzzling scenario, revealing that specific fish parasites reduce their risk to go co-extinct by using hosts with low vulnerability (Fig. 4). The authors used a large dataset of host parasite interactions, and computed the vulnerability of each parasite as the product of the vulnerability of its hosts. Then, they compared the average vulnerability of the hosts used by each parasite with the parasite's specificity, finding an inverse relationship. These results indicate that the more specific a parasite is, the less vulnerable are, on average, its hosts (Fig. 5), and that host–parasite networks could be particularly robust towards host extinctions. Investigating a highly resolved fish-parasite network in the Upper Paraná River floodplain, Dallas and Cornelius (2015) provided additional support to this idea. In particular, they found that a sequence of host extinctions based on fish vulnerability leads to a slower decline in parasite diversity compared to several other scenarios of non-random biodiversity loss.
Fig. 4.
Schematic representation of how parasite specificity and host vulnerability may contribute to the structure of fish-parasite networks according to Strona et al. (2013). Specialist parasites tend to use hosts that are easy to encounter, i.e. hosts (i) persisting over long evolutionary time, (ii) broadly distributed, and/or (iii) locally abundant. These properties are inversely related with host vulnerability to extinction. The presence of a gradient in parasite host specificity could promote the emergence of a nested structure.
Fig. 5.
Graph showing the relationship between fish parasite specificity and the corresponding average vulnerability of the hosts used by those parasites. Data were obtained using the same data and procedure as in Strona et al. (2013), computing mean host vulnerability values for different parasite host range classes. Differently from Strona et al. (2013), however, classes were defined using a logarithmic progression instead of a geometric one, resulting in an even tighter relationship between log(host range) and mean host vulnerability (rs = 0.93; p < 0.05).
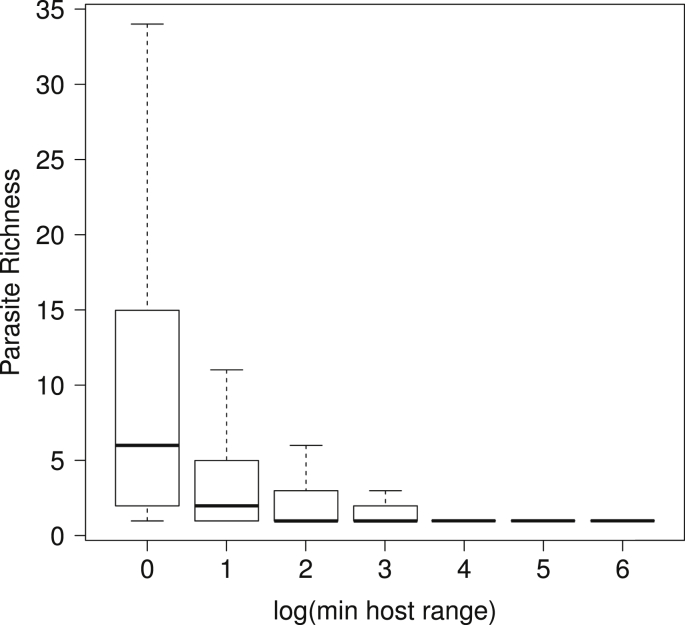
Strona et al. (2013) suggested a simple and intuitive explanation to the pattern shown in Fig. 5. The evolution of specialization requires repeated encounters between a host and a parasite. It is therefore likely that a parasite will become specialized on readily available hosts, i.e. hosts having wide distribution, high local abundance and persistence over evolutionary times. In principle, all of these properties are typical of hosts with low vulnerability to extinction, which explains the observed relationship. Conversely, a host having narrow distribution or small populations would be a challenging benchmark for the evolution of specialized interactions, and thus would probably be targeted by generalist parasites. This mechanism would promote a higher parasite richness on hosts used by specialists, producing a well-known pattern known as asymmetry of interactions (see Fig. 6 and, for example, Kelley and Farrell, 1998, Vázquez et al., 2005, Vázquez et al., 2007).
Fig. 6.
Example of asymmetry of interactions as observed in all host parasite records available from FishPest dataset (Strona and Lafferty, 2012). The graph shows the relationship between the maximum specificity of the parasites using a certain host species, and the parasite richness on that host species. Boxplots correspond to different classes of hosts identified on the basis of the maximum specificity of their parasites. Thus, the first boxplot provides information on parasite species richness of all fish species whose most specific parasite uses just one host. It is apparent that specific parasites tend to use hosts harboring many parasites, while species-poor parasitofaunas are often composed by generalist parasites. Boxes indicate first and third quartiles, whiskers indicate range values, and horizontal lines indicate median values.
In principle, the same mechanism could be also responsible for the emergence of nested patterns, i.e. situations where the set of hosts used by any parasite is a subsample of the set of hosts used by any other more generalist parasite (Fig. 4). Yet, it should be highlighted that there is still no consensus whether nestedness is typical or not of host parasite networks, mostly due to the different level of organization (infra-, component, compound) at which the community is considered, and to the different methods used to measure nestedness and assess its significance (Poulin, 1996, Rohde et al., 1998, Poulin and Guégan, 2000, Fortuna et al., 2010, McQuaid and Britton, 2013a, Strona and Fattorini, 2014c, Strona and Veech, 2015).
5. Ecosystem implications
Understanding why parasites are important for ecosystems is counter-intuitive. It has been argued that parasites play roles in ecosystems similar to those played by predators. This comparison embraces some relevant aspects of the way parasites can affect ecosystems (see Raffel et al., 2008 for a review). For example, as discussed below, parasites could be important in keeping host populations from growing too much (Combes, 1996). Moreover, several parasites have evolved complex strategies aimed at maximizing their fitness, possibly due to arms race processes comparable to those observed between predators and prey (Dawkins and Krebs, 1979). Yet, besides the similarities, there are also fundamental differences between parasites and predators, the most obvious one being that predators are usually larger than their prey (Cohen et al., 1993), while parasites are, in general, smaller than their hosts (Combes, 2005).
Parasites often dominate ecosystems not only in terms of diversity, but also in terms of biomass (Kuris et al., 2008). They are present throughout the whole food web, and parasites having complex life cycles often exploit hosts at different trophic levels in a single lifetime, in order to develop and reproduce. In doing this, they may increase the stability of the whole network by increasing connectance and nestedness (Lafferty et al., 2006). Consequently, parasite diversity and abundance can be marks of ecosystem health (Hudson et al., 2006, Kuris et al., 2008).
Parasites affect ecosystems in complex ways (see Hatcher et al., 2006 for a detailed review). For example, a parasite infecting two hosts using the same resource/s may reduce their competition by limiting their population sizes, preventing the stronger species from outcompeting the weaker one. Ideally, the ‘control’ parasites exert on host density may increase the overall resource availability, possibly promoting species coexistence (see Fig. 1b in Hatcher et al., 2006). This scenario, however, assumes the existence of a feedback mediated equilibrium keeping host density at a level low enough to promote biodiversity and to reduce the risk of parasite outbreaks, but still sufficient to sustain parasite populations. Deviations from this equilibrium due, for example, to human driven ecosystem alterations may have dramatic, unpredictable effects.
In particular, the decline in free-living populations can drive a parasite to local extinction long before the extinction of all of its hosts, by reducing the parasite's probability to find and infect a suitable host (Altizer and Pedersen, 2008). Although the release from parasites may slow down the decline of free-living populations, its effect is likely negligible in face of the disturbance responsible for the decline in the first place.
Understanding how parasites can respond to host population decline is central to the study of co-extinctions. In general, the effect of the reduction in host population density on parasite infection dynamics can be partially compared to that of habitat fragmentation on the dispersal-colonization-extinction patterns of free-living species. Thus, it may benefit from techniques typical of metacommunity modeling (Mihaljevic, 2012). Yet, investigating co-extinctions under the perspective of extinction cascades, i.e. assuming that a parasite will go extinct following its hosts, is still preponderant (see, for example, Dallas and Cornelius, 2015), even if this corresponds to the unrealistic idea that a free-living species will survive until the complete depletion of its habitat. Studies based on the comparison of parasite diversity between hosts having different threat status (such as Powell, 2011) can provide some interesting insights. Nevertheless, they could be biased by the underlying assumption that the threat status of a host is completely independent from the evolutionary processes responsible for its parasite richness, which is not necessarily true (see the next section, and Strona and Fattorini, 2015).
6. Current risk
Understanding why the number of observed co-extinctions is unexpectedly low (Dunn et al., 2009) is much important. The co-evolutionary hypothesis suggested by fish parasites (see paragraph 4) provides a reasonable solution to the issue, by explaining how the way co-evolution has shaped host–parasite networks can make them robust towards (non-random) species loss. Yet, this mechanism does not imply that extant parasite species are not endangered, since the vulnerability of a species in an evolutionary perspective does not necessarily coincide with its current extinction risk. The evolutionary vulnerability of a species is the result of an adaptive path that has lasted millions of years, eventually shaping the species' ability to survive under certain circumstances. By contrast, the current extinction risk of a species is determined by several factors, such as habitat degradation, over-exploitation, and climate change, that are often independent from the species' evolutionary history (Owens and Bennett, 2000, Thomas et al., 2004). The deeper the discrepancies between hosts' evolutionary vulnerability and their actual extinction risk, the lower the chances that the structure of interaction networks shaped by co-evolution will protect parasites from co-extinctions. To my knowledge, however, this important aspect has been largely ignored, even if it has primary implications for both designing analyses and interpreting results.
Strona et al. (2013) were mainly interested in understanding how specialization could be so common in parasites given the possible increased risk of extinction for specialists, hence focusing on co-evolutionary dynamics. Since they needed to assess evolutionary vulnerability, they used host life-history traits as a proxy (Froese and Pauly, 2011). Yet, this measure of ‘intrinsic’ vulnerability is not necessarily consistent with the actual extinction risk of a species as indicated, for example, by IUCN (Strona, 2014). Thus, the pattern observed for fish may imply that, although co-evolutionary mechanisms promoting parasite persistence have been effective up to date, they may fail in the future due to the fact that human activities and climatic change are driving species to extinction with little consistency in respect to their evolutionary history. Although sharks are among the most old and successful fish group, not having shown any sign of declining during the last 65 million years, they are now much endangered by overfishing, low prey availability and finning (Bradley and Gaines, 2014). The same holds for big-game species: the high extinction risk of the host-specific rhino bot fly is the result of poaching (Brown and Layton, 2001), and has little to do with the evolution and maintenance of specialization, and its possible condition of evolutionary dead end (see, for example, Futuyma and Moreno, 1988, Moran, 1988, Wiegmann et al., 1993, Whitlock, 1996, Kelley and Farrell, 1998, Forister et al., 2012).
A recent study focusing on terrestrial vertebrates (carnivores and ungulates) found that hosts indicated by IUCN at extinction risk harbor less multi-host parasites than not endangered hosts (Farrell et al., 2015). The authors of this study argue that this tendency, at least for ungulates, emerges from a disproportionate loss of generalist parasites in threatened hosts, likely due to the effect of a reduction in contacts between hosts (caused, in turn, by decreasing population size, fragmentation, etc.). The work by Farrell et al. (2015) has some major limitations in its theoretical and methodological approach, which cast doubts on both the results and their interpretation, and that are discussed in detail in Strona and Fattorini (2015). Nevertheless, it has the merit of introducing the important idea that generalist parasites could be more affected by future species loss than specialist ones, which I will discuss more thoroughly in the next section.
7. Specialization/generalism tradeoffs
Considering specialization as the main factor determining co-extinction risk could be imprecise, since (i) co-evolution can reduce the extinction risk for specialists (Strona et al., 2013), and (ii) generalists can be at risk due to current scenarios of fragmentation, habitat loss, and the consequent reduction in host geographical range and population sizes (Farrell et al., 2015). The latter aspect, although fundamental, has been largely unexplored.
There are pros and cons in “choosing” specialization over generalism (Joshi and Thompson, 1995), as summarized by the “jack of all trades, master of none” hypothesis, which states that evolutionary tradeoffs exist between the ability of a species to use many resources, and its efficiency in using each of them (Kelley and Farrell, 1998). This implies that a specialist should be able to outcompete a generalist in the use of a particular resource under limiting conditions. Conversely, a generalist should be able to compensate this disadvantage in at least two ways, namely having higher chances of finding useful resources, and having a higher ability to switch from a resource to another in case the first becomes poorly available.
Thus, when both a generalist and a specialist have only a few resources available, the trade-off will disfavor the first. It is not clear what will happen with future scenarios of climatic change, yet it is reasonable to assume that we will experience an overall loss of biodiversity (Bellard et al., 2012). In this context, the specialist parasites that will not go extinct will probably be less affected than generalist ones by the overall reduction in host availability.
Ideally, we could verify this by focusing on host co-occurrence, assuming that future reductions in geographical range overlaps between host species will be detrimental to generalist parasites. The current increasing availability of global species distribution data, climatic models, host–parasite databases, and analytical techniques to measure species co-occurrence (Veech, 2013) offers a unique opportunity to investigate these issues at large scale.
8. Complex life-cycles
Many parasite species use different hosts (with different levels of specificity) at different development stages (Adamson and Caira, 1994). Understanding the complexity of parasitic life cycles is fundamental to investigate co-extinction risk, since the loss of a key intermediate host could be enough to drive a parasite to extinction (Lafferty, 2012).
In order to get a conservative estimate of extinction risk for a parasite having a complex life cycle, one should consider both the vulnerability of each host at the various developmental stages of a parasite, and the effectiveness of the parasite transmission processes. This last aspect is conceptually similar to the importance for a generalist parasite to have access to the full set of its potential hosts. Yet, the disruption of a single link in the transmission chain can reduce the fitness of a complex life cycle parasite to zero. This effect is quite difficult to be quantified due to the lack of precise data about intermediate hosts for many parasite species. Considering the fundamental role parasites play in food webs, we may expect that the severe alterations that are affecting trophic networks in most ecosystems (Walther et al., 2002) will have (and perhaps have already had) dramatic effects on parasite diversity. Again, examining the variation in range overlap between host species used by different developmental stages of a parasite could provide some hints about possible future scenarios of parasite diversity loss.
9. Host finding strategies
Future changes in host species geographical ranges could have negative effects on parasites, both by reducing the set of hosts available to generalist parasites, and by breaking up some of the trophic links needed to complete complex life-cycles. However, current scenarios of disturbance of natural ecosystems may have another, largely neglected, negative effect on parasite communities. Many parasites have evolved complex host finding strategies (Haas, 2003, Strona, 2015), relying not only on host chemical clues, but also on host behaviors and/or host interactions (Strona, 2015). Alterations in host ecology, such as changes in foraging strategy (Traill et al., 2010) and/or behaviors (Sih, 2013), may negatively affect the ability of a parasite to find a suitable host. This could be particularly compelling for highly specialized parasites, both because they could be no longer able to recognize hosts with altered behaviors, or because modifications in habitat use (at a local scale) could strongly reduce contacts between hosts and parasites. The possible competitive advantage of hosts having altered behavior and/or foraging strategy deriving from parasite release could discourage them from getting back to their old behavior/strategy, thus driving to extinction those parasites unable to adapt to the new settings.
In addition, a recent study highlighted how predatory activity could be important for the transmission of single-host parasites using both predators and their prey (Strona, 2015). This counterintuitive observation could have strong implications for conservation, since the loss of either a prey or its predator could have a detrimental effect on a parasites using both (Strona, 2015).
Since our knowledge about host finding strategies is limited, it is hard to make predictions for the future. All of this could be complicated by the commonness of parasitic manipulation, which creates very intricate scenarios (Hughes et al., 2012).
10. Biological invasions
The colonization of new areas by alien species represents a major threat to biodiversity (Mooney and Cleland, 2001, Clavero and Garcia-Berthou, 2005). It has been argued that the success of an invasion can be affected at various degrees by parasites (see, for example, Torchin et al., 2003, Colautti et al., 2004, Heger and Jeschke, 2014).
On the one hand, invaders can lose their parasites during translocation. This can happen because: (i) the migrants are uninfected (as in the case of fish or other marine organisms moving as larvae); (ii) the alien population is not big enough to maintain parasites during the first phases of the invasion; or (iii) some of the intermediate hosts required by the invaders' parasites are not available in the new area (Torchin and Mitchell, 2004). On the other hand, invaders can establish symbiotic relationships with parasites from native populations (Prenter et al., 2004). The latter case can produce different outcomes. For example, the lack of co-evolution may lead to a high virulence of native parasites against the alien invaders (Little et al., 2010). Alternatively, invaders could be immune to native parasites. In this case, they could even convey benefits to the free-living natives by acting as resistant targets for their parasites, thus reducing the density of the infectious transmission stages through a ‘dilution effect’ (Kopp and Jokela, 2007).
Obviously, these processes are not mutually exclusive, i.e. invaders can lose some parasites during translocation and get some other ones from the native population, even if our knowledge on this subject is still limited, and previous studies have provided contrasting evidence (Colautti et al., 2004). However, a part from the effects on hosts, it is interesting to ask what could be the effect of biological invasions on parasite co-extinctions.
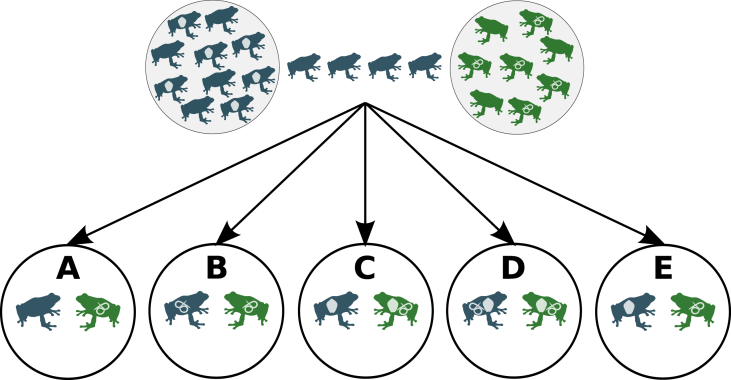
Fig. 7 simplifies some of the different possible outcomes of an invasion where individuals of a species parasitized in its native range move to a new area:
-
1)
Invaders lose their parasites, and do not get new parasites from local hosts (Fig. 7A). In this case, invaders' parasites will not experience any benefit, since they will be confined to their original distribution; conversely, native parasites (i.e. those of the newly colonized area) will be negatively affected, since invaders (taking advantage from parasite release) could reduce native host populations, possibly driving their parasites to extinction.
-
2)
Invaders lose their parasites and get new ones from local hosts (Fig. 7B). This will have a positive effect for local parasites, since they will expand their host range by incorporating the alien species. Moreover, native parasites will also affect negatively invaders' fitness, thus reducing their chances to outcompete native hosts.
-
3)
Invaders retain their parasites, and these establish new symbioses with local species (Fig. 7C). In this situation, alien parasites will benefit from expanding their host range; at the same time, they will help their original hosts (i.e. the invaders) to succeed in the colonization of the new area by affecting negatively local hosts (i.e. the invaders' potential competitors). However, alien parasites will also continue to affect their original hosts (i.e. the invaders), possibly preventing them to outcompete native hosts. If invaders are also infected by parasites from native hosts (Fig. 7D), this could favor native species both by limiting invaders' population growth, and by reducing the average parasitic load through dilution.
-
4)
Invaders do not lose their parasites, they do not get new ones from native hosts, and their parasites do not expand their host range (Fig. 7E). In this case, alien parasites will have an advantage due to the expansion of their hosts' geographical range. The negative effect of alien parasites on invaders will reduce the risk they will outcompete native hosts and drive local parasites to extinction.
Fig. 7.
Schematic representation of the possible different parasitological consequences of a biological invasion. A: The invader loses its parasite and does not get local parasites; B: The invader loses its parasites and gets new ones from native hosts; C: The invader retains its parasites and these establish new symbioses with local species; D: The invader retains its parasites and acquire new parasites from local hosts; its parasites establish new symbioses with local host species; E: The invader does not lose its parasites, does not get new ones from native hosts, and its parasites do not expand their host range.
In general, also parasites lost by invaders will benefit from the geographical range expansion of their hosts, since this will provide them chances for future colonization. A parasite that has not succeeded in an invasion (for example due to a too small founding population), could get successfully established in the future if carried to the new area by a subsequent set of invading hosts.
11. Future directions (with an eye on the past)
Insights into current co-extinction trajectories could be obtained only through long term monitoring of host/parasite population dynamics. However, even with this approach, the complexity of possible outcomes of direct and indirect interactions could pose caveats on the interpretation of results, making it difficult to draw general conclusions. The evolutionary time scale of co-extinction processes clearly prevents from setting up experiments. A possible, promising alternative is looking back to the past.
Combining ancient DNA and microscopic analyses of coprolites from New Zealand moa (Aves: Dinornithiformes), Wood et al. (2013) have demonstrated the potential of studying parasite assemblages from extinct clades, providing some information to answer the question whether or not the extinction of moas (that dates back a century) led to the co-extinction of their parasites (Bush and Kennedy, 1994).
On the one hand, the authors found that the parasitofauna of extinct moas is highly consistent with that of extant New Zealand ratites, which could suggest it survived extinction. On the other hand, many of the parasites they found on moa appeared to be host specific, which may depict different scenarios. Although further investigation is needed to solve this issue, the study demonstrates how this kind of approach, once extended to other groups, could provide a unique chance to obtain empirical information about co-extinction events, possibly offering a glimpse of the future.
12. Mathematical modeling and digital evolving organisms
The most obvious alternative approach to investigate co-extinctions is using mathematical modeling, which, however, poses many challenges (Bellomo and Carbonaro, 2011). In particular, as regarding for models including the effect of parasites on host interactions (i.e. competition and/or predation), focusing on several species or adding realistic life-history assumptions makes the mathematical approach analytically intractable (Dunn and Hatcher, 2015).
One of the most popular co-evolutionary model is Webworld (Caldarelli et al., 1998). In Webworld, species co-evolve generating trophic webs. For this, a random set of features corresponding to offense and defense abilities is attributed to each species. The model uses a system of differential equations, and evolves thanks to mutations altering one feature of a given species at a time (Caldarelli et al., 1998). This kind of approach could be extended to model host–parasite co-evolution, and actually some attempts have been made (McQuaid and Britton, 2013a, McQuaid and Britton, 2013b), even if they have received little attention. A major limitation of this kind of approaches (and of others focusing on host–parasite co-evolutions, see Best et al., 2009, Best et al., 2010) is that they work at the species level, due both to conceptual (they are based on population dynamic models) and computational issues. However, since natural selection operates at the individual level, important caveats apply to the interpretation of results produced by Webworld-like models (Chowdhury and Stauffer, 2005). A possible, alternative solution to this issue is using artificial life simulations, such as Avida (Ofria and Wilke, 2004).
Avida is a complex platform for artificial life simulations that has already permitted to tackle some very important evolutionary issues, such as the mechanisms promoting the evolution of complex features (Lenski et al., 2003). Differently from Webworld-like models, organisms in Avida (‘Avidians’) are treated individually. Preliminary studies have demonstrated that using digital evolving organisms has a high potential to answer important questions in the field of host–parasite coevolution. For example, Zaman et al. (2011) have shown that host–parasite co-evolution can promote and maintain species diversity, while Zaman et al. (2014) have demonstrated that host–parasite interactions can promote the evolution of complex features even in case these are not rewarded (in terms of reproductive fitness) more than simple features.
In artificial life simulations, the status of host and parasite populations can be continuously monitored. This permits to track species declines, and to record the occurrence and the exact timing of host and parasite extinction events, offering a unique opportunity to investigate the dynamics and the causes of co-extinctions. I am not the first to emphasize the potential relevance of artificial life simulations to the field of ecological networks (see Fortuna et al., 2013). Yet, a part from Zaman et al. papers (Zaman et al., 2011, Zaman et al., 2014), this approach has received very little attention to date.
13. Lessons from eradication attempts
The main difference between parasite loss following host extinction, and human eradication of harmful pathogens is that the latter aims at eliminating parasites to preserve their hosts (and often in face of an increasing host availability). Nevertheless, eradication attempts can provide important insights into co-extinctions, since strategies such as the elimination of potential vectors, or the immunization of final hosts can have effects on parasite populations similar to those produced by biodiversity loss.
The global geography of infectious diseases is strongly associated with the distribution of richness (Bonds et al., 2010), with poor countries being the most affected (Jones et al., 2008). More than 1 billion people in developing countries is parasitized by helminths. Conversely, people living in developed countries are virtually helminth-free, besides being exposed to a much lower microbial burden than people from developing areas (Yazdanbakhsh et al., 2002, Weinstock and Elliott, 2009). This may indicate that parasite control programs can be locally effective if conducted properly (Bowman, 2006). Yet, if we focus on globally widespread diseases of primary concern, it looks like eradication, i.e. the worldwide interruption of disease transmission, although feasible (Bowman, 2006), is a very hard task (Hopkins, 2013).
For example, despite the great effort spent since the early days of the World Health Organization's Global Malaria Eradication Program (GMEP) in the late 1950s, and highly intensified over the past decade, in the only 2009 there were more than 781,000 malaria-related deaths (Alonso et al., 2011). Similarly, there are about 200 million people infected globally by schistosoma, and nearly 800 millions at risk. Conversely, the cases of dracunculiasis are decreasing year after year thanks to a very effective global control program that started in 1986, and that aims at reaching the complete eradication by the end of 2015 (Cavendish, 2014).
A recent study demonstrated that the reintroduction of a native river prawn that preys on snail intermediate hosts has the potential to eliminate (locally) schistosomiasis if paired with drug distribution campaigns (Sokolow et al., 2015). This may suggest that, despite human driven reduction of diversity can lead to parasite loss (for example, species belonging to the genus of the above mentioned native prawn, Macrobrachium spp., are known to carry a variety of parasites, see Brock, 1993), it may disproportionately favor the spread of some pathogens. In particular, changes in predator communities may facilitate the emergence of zoonotic diseases carried by hosts from lower trophic levels (Levi et al., 2012).
As regards generalist parasites, predicting the effect of ecosystem alterations on the dynamics of host–parasite infections can be very difficult. For example, in some cases the abundance of available hosts, instead of triggering outbreaks, may generate a dilution effect, reducing the overall parasite burden (and potential risk for human transmission) (Schmidt and Ostfeld, 2001, Perkins et al., 2006). Moreover, there are fundamental aspects about the role played by parasite diversity in infection processes that are still largely unexplored. It has been shown that the presence of certain parasite species may significantly increase or decrease host susceptibility towards other parasites, as suggested by patterns of non-random (positive and negative) species co-occurrence in parasite infracommunities (Telfer et al., 2010). Besides, although the benefits of the eradication of harmful pathogens are hardly debatable (Bowman, 2006, Cavendish, 2014), it is interesting that the very low exposure to pathogens in developed countries has been suggested as potentially associated to an increase in immunological diseases (according to the so called “hygiene hypothesis”, Strachan, 1989). Even more interestingly, this has recently led to the development of experimental treatments of autoimmune disorders (such as multiple sclerosis) based on helminth administration (Fleming, 2013).
These aspects could offer fundamental insights for the development of control strategies. More than this, they highlight how preserving both host and parasite diversity is not just a matter of equal rights or numbers, but is key to the future of human and wildlife health.
14. Concluding remarks
Despite their fundamental importance in the maintenance of ecosystem stability and diversity, parasites have had a hard time catching the interest of conservation biologists. However, things are now changing, and this is by no means the first review on the subject (see, for example, Altizer et al., 2007, Dunn, 2009, Dunn et al., 2009, Lafferty, 2012). Yet, despite the increasing number of papers focusing on host–parasite co-extinctions, we are still in a very preliminary phase of our exploration of the subject. This emerges clearly from the inconsistencies between different analyses, and even more clearly from models. The main message from Lafferty's simulations (2012) is that co-extinctions can take several different directions depending on equally sound starting assumptions, and that generalizing mechanisms (and hence predictions) is, in most cases, unfeasible.
When I committed to write this paper, my aim was summarizing some of the main aspects contributing to this complexity, focusing, in particular, on the indirect effects parasites can have on hosts and, more in general, on ecosystems. I have done my best to accomplish this, but still I feel like I have left readers with only a glimpse of the overwhelming complexity lying behind host–parasite co-extinctions. Nevertheless, I hope this review could provide a logical framework for future work, in particular by drawing a clear conceptual distinction between (i) the co-evolutionary processes that have shaped host–parasite networks throughout millions of years, (ii) the ecological processes that have continuously been controlling such co-evolutionary dynamics, and (iii) what is happening now to those networks, which is a combination of the effect of human alterations and ecological responses.
Before attempting at integrating these three compartments, we probably need a more thorough understanding of each of them. Focusing on what happens next makes little sense without a clear grasp on how things have got to this point. The blazing speed by which we are losing biodiversity often forces us to focus on current patterns, looking for possible disrupting mechanisms. I am not criticizing this kind of approach, since it is often the only one possible when time is running out and conservation actions are needed. Yet, I would recommend that at least theoreticians focus first on the mechanisms that have generated the present patterns, before searching for the easiest paths to disassemble them. This would highlight, for example, how considering specialists as much more endangered than generalists is in obvious contrast with the predominance of specialization in the real world ecological networks (Strona and Veech, 2015). Focusing on the co-evolutionary paths leading to specialization could provide some unexpected results, and perhaps offer a broader perspective on what to expect for the future.
What is becoming more and more clear, is that the potential future loss of parasites could have an enormous impact on free-living species. Despite their recent popularity, this ideas have empirical roots dating back almost twenty years. Hudson et al. (1998) have provided a remarkable example, by showing that cyclic fluctuations (and crashes) commonly observed in red grouse populations are induced by parasites, and disappear when parasites are removed by just 20% of hosts. Extending these results at the community (and meta-community) level is clearly out of empirical reach, and far from being analytically tractable. Yet, it is intuitive how the implications of this discovery go beyond the population level. Focusing only on how the loss of free-living species can cause parasite co-extinction could offer a biased perspective. Considering that there are several convincing answers to the question “why should we care about parasites?”, perhaps it is time to ask: what happens when all parasites are lost?
Acknowledgments
I would like to thank Prof. Andrew Thompson and the organizers of the IECID 2015 conference for giving me the opportunity to write this review. I am indebted with two anonymous referees and Simone Fattorini for their useful suggestions on a previous version of this paper. The views expressed are purely those of the writers and may not, in any circumstances, be regarded as stating an official position of the European Commission.
References
- Adamson M.L., Caira J.N. Evolutionary factors influencing the nature of parasite specificity. Parasitology. 1994;109(S1):S85–S95. doi: 10.1017/s0031182000085103. [DOI] [PubMed] [Google Scholar]
- Alonso P.L., Brown G., Arevalo-Herrera M., Binka F., Chitnis C., Collins F., Doumbo O.K., Greenwood B., Hall B.F., Levine M.M., Mendis K., Newman R.D., Plowe C.V., Rodríguez M.H., Sinden R., Slutsker L., Tanner M. A research agenda to underpin malaria eradication. PLoS Med. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S., Nunn C.L., Lindenfors P. Do threatened hosts have fewer parasites? A comparative study in primates. J. Anim. Ecol. 2007;76:304–314. doi: 10.1111/j.1365-2656.2007.01214.x. [DOI] [PubMed] [Google Scholar]
- Altizer S., Pedersen A.B. Host-pathogen evolution, biodiversity and disease risks for natural populations. In: Carroll S.P., Fox C.W., editors. Conservation Biology: Evolution in Action. Oxford University Press; Oxford: 2008. pp. 259–277. [Google Scholar]
- Bellard C., Bertelsmeier C., Leadley P., Thuiller W., Courchamp F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012;15:365–377. doi: 10.1111/j.1461-0248.2011.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo N., Carbonaro B. Toward a mathematical theory of living systems focusing on developmental biology and evolution: a review and perspectives. Phys. Life Rev. 2011;8:1–18. doi: 10.1016/j.plrev.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Best A., White A., Boots M. The implications of coevolutionary dynamics to host–parasite interactions. Am. Nat. 2009;173:779–791. doi: 10.1086/598494. [DOI] [PubMed] [Google Scholar]
- Best A., White A., Kisdi E., Antonovics J., Brockhurst M.A., Boots M. The evolution of host–parasite range. Am. Nat. 2010;176:63–71. doi: 10.1086/653002. [DOI] [PubMed] [Google Scholar]
- Bonds M.H., Keenan D.C., Rohani P., Sachs J.D. Poverty trap formed by the ecology of infectious diseases. Proc. R. Soc. B. 2010;277:1185–1192. doi: 10.1098/rspb.2009.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrvall C., Ebenman B. Early onset of secondary extinctions in ecological communities following the loss of top predators. Ecol. Lett. 2006;9:435–442. doi: 10.1111/j.1461-0248.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- Bowman D.D. Successful and currently ongoing parasite eradication programs. Vet. Parasitol. 2006;139:293–307. doi: 10.1016/j.vetpar.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Bradley D., Gaines S.D. Counting the cost of overfishing on sharks and rays. eLife. 2014;3:e02199. doi: 10.7554/eLife.02199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J.A. A synopsis of pathology, diseases, and production problems of cultured Macrobrachium, with an emphasis on experiences in Hawaiian prawn farming. In: McVey J.P., editor. vol. I. CRC Press; 1993. pp. 361–391. (CRC Handbook of Mariculture). [Google Scholar]
- Brown G., Layton D.F. Cambridge University Press; Cambridge: 2001. A Market Solution for Preserving Biodiversity: the Black Rhino. [Google Scholar]
- Bush A.O., Kennedy C.R. Host fragmentation and helminth parasites: hedging your bets against extinction. Int. J. Parasitol. 1994;24:1333–1343. doi: 10.1016/0020-7519(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Caldarelli G., Higgs P.G., McKane A.J. Modelling coevolution in multispecies communities. J. Theor. Biol. 1998;193:345–358. doi: 10.1006/jtbi.1998.0706. [DOI] [PubMed] [Google Scholar]
- Cavendish J. The last bastions of Guinea-worm disease. Bull. World Health Organ. 2014;92:854–855. doi: 10.2471/BLT.14.021214. World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury D., Stauffer D. Evolutionary ecology in silico: does mathematical modelling help in understanding ‘generic’trends? J. Biosci. 2005;30:277–287. doi: 10.1007/BF02703709. [DOI] [PubMed] [Google Scholar]
- Clavero M., Garcia-Berthou E. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 2005;20:110. doi: 10.1016/j.tree.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Clayton D.H., Price R.D. Taxonomy of New World Columbicola (Phthiraptera: Philopteridae) from the Columbiformes (Aves), with descriptions of five new species. Ann. Entomol. Soc. Am. 1999;92:675–685. [PubMed] [Google Scholar]
- Cohen J.E., Pimm S.L., Yodzis P., Saldaña J. Body sizes of animal predators and animal prey in food webs. J. Anim. Ecol. 1993;62:67–78. [Google Scholar]
- Colautti R.I., Ricciardi A., Grigorovich I.A., MacIsaac H.J. Is invasion success explained by the enemy release hypothesis? Ecol. Lett. 2004;7:721–733. [Google Scholar]
- Combes C. Parasites, biodiversity and ecosystem stability. Biodivers. Conserv. 1996;5:953–962. [Google Scholar]
- Combes C. University of Chicago Press; Chicago and London: 2005. The Art of Being a Parasite. [Google Scholar]
- Costello M.J., May R.M., Stork N.E. Can we name earth's species before they go extinct? Science. 2013;339:413–416. doi: 10.1126/science.1230318. [DOI] [PubMed] [Google Scholar]
- Dallas T., Cornelius E. Co-extinction in a host-parasite network: identifying key hosts for network stability. Sci. Rep. 2015;5:13185. doi: 10.1038/srep13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins R., Krebs J.R. Arms races between and within species. P. Roy. Soc. B Biol. Sci. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. [DOI] [PubMed] [Google Scholar]
- Diamond J.M. Overview of recent extinctions. In: Western D., Pearl M., editors. Conservation for the Twenty-first Century. Oxford University Press; Oxford: 1989. pp. 37–41. [Google Scholar]
- Dobson A., Lafferty K.D., Kuris A.M., Hechinger R.F., Jetz W. Homage to Linnaeus: how many parasites? How many hosts? Proc. Natl. Acad. Sci. U. S. A. 2008;105:11482–11489. doi: 10.1073/pnas.0803232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R.R. Modern insect extinctions, the neglected majority. Conserv. Biol. 2005;19:1030–1036. [Google Scholar]
- Dunn R.R. Coextinction: anecdotes, models, and speculation. In: Turvey S.T., editor. Holocene Extinctions. Oxford University Press; Oxford: 2009. pp. 167–180. [Google Scholar]
- Dunn R.R., Harris N.C., Colwell R.K., Koh L.P., Sodhi N.S. The sixth mass coextinction: are most endangered species parasites and mutualists? Proc. Roy. Soc. B Biol. Sci. 2009;276:3037–3045. doi: 10.1098/rspb.2009.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A.M., Hatcher M.J. Parasites and biological invasions: parallels, interactions, and control. Trends Parasitol. 2015;31:189–199. doi: 10.1016/j.pt.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Eklöf A., Ebenman B.O. Species loss and secondary extinctions in simple and complex model communities. J. Anim. Ecol. 2006;75:239–246. doi: 10.1111/j.1365-2656.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- Farrell M.J., Stephens P.R., Berrang-Ford L., Gittleman J.L., Davies T.J. The path to host extinction can lead to loss of generalist parasites. J. Anim. Ecol. 2015;84:978–984. doi: 10.1111/1365-2656.12342. [DOI] [PubMed] [Google Scholar]
- Fleming J.O. Helminth therapy and multiple sclerosis. Int. J. Parasitol. 2013;43:259–274. doi: 10.1016/j.ijpara.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Forister M.L., Dyer L.A., Singer M.S., Stireman J.O., III, Lill J.T. Revisiting the evolution of ecological specialization, with emphasis on insect-plant interactions. Ecology. 2012;93:981–991. doi: 10.1890/11-0650.1. [DOI] [PubMed] [Google Scholar]
- Fortuna M.A., Stouffer D.B., Olesen J.M., Jordano P., Mouillot D., Krasnov B.R., Poulin R., Bascompte J. Nestedness versus modularity in ecological networks: two sides of the same coin? J. Anim. Ecol. 2010;79:811–817. doi: 10.1111/j.1365-2656.2010.01688.x. [DOI] [PubMed] [Google Scholar]
- Fortuna M.A., Zaman L., Wagner A.P., Ofria C. Evolving digital ecological networks. PLoS Comput. Biol. 2013;9:e1002928. doi: 10.1371/journal.pcbi.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froese R., Pauly D. World Wide Web Electronic Publication; 2011. FishBase.http://www.fishbase.org (accessed on 17.11.14) [Google Scholar]
- Futuyma D.J., Moreno G. The evolution of ecological specialization. Annu. Rev. Ecol. S. 1988;19:207–233. [Google Scholar]
- Haas W. Parasitic worms: strategies of host finding, recognition and invasion. Zoology. 2003;106:349–364. doi: 10.1078/0944-2006-00125. [DOI] [PubMed] [Google Scholar]
- Hatcher M.J., Dick J.T., Dunn A.M. How parasites affect interactions between competitors and predators. Ecol. Lett. 2006;9:1253–1271. doi: 10.1111/j.1461-0248.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- Heger T., Jeschke J.M. The enemy release hypothesis as a hierarchy of hypotheses. Oikos. 2014;123:741–750. [Google Scholar]
- Hopkins D.R. Disease eradication. N. Engl. J. Med. 2013;368:54–63. doi: 10.1056/NEJMra1200391. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Dobson A.P., Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Dobson A.P., Lafferty K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006;21:381–385. doi: 10.1016/j.tree.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Hughes D.P., Brodeur J., Thomas F. Oxford University Press; Oxford: 2012. Host Manipulation by Parasites. [Google Scholar]
- Huyse T., Webster B.L., Geldof S., Stothard J.R., Diaw O.T., Polman K., Rollinson D. Bidirectional introgressive hybridization between a cattle and human Schistosome species. PLoS Pathog. 2009;5:e1000571. doi: 10.1371/journal.ppat.1000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN . 2014. The IUCN Red List of Threatened Species. Version 2014.3.http://www.iucnredlist.org (accessed on 17.11.14) [Google Scholar]
- Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen D. Conservation implications of parasite co-reintroduction. Conserv. Biol. 2015;29:602–604. doi: 10.1111/cobi.12421. [DOI] [PubMed] [Google Scholar]
- Joshi A., Thompson J.N. Trade-offs and the evolution of host specialization. Evol. Ecol. 1995;9:82–92. [Google Scholar]
- Kearn G.C. Evolutionary expansion of the Monogenea. Int. J. Parasitol. 1994;24:1227–1271. doi: 10.1016/0020-7519(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Kellert S.R. Island Press; Washington: 1997. The Value of Life: Biological Diversity and Human Society. [Google Scholar]
- Kelley S.T., Farrell B.D. Is specialization a dead end? The phylogeny of host use in Dendroctonus bark beetles (Scolytidae) Evolution. 1998;52:1731–1743. doi: 10.1111/j.1558-5646.1998.tb02253.x. [DOI] [PubMed] [Google Scholar]
- Kerr J.T., Currie D.J. Effects of human activity on global extinction risk. Conserv. Biol. 1995;9:1528–1538. [Google Scholar]
- Koh L.P., Dunn R.R., Sodhi N.S., Colwell R.K., Proctor H.C., Smith V.S. Species coextinctions and the biodiversity crisis. Science. 2004;305:1632–1634. doi: 10.1126/science.1101101. [DOI] [PubMed] [Google Scholar]
- Koh L.P., Sodhi N.S., Brook B.W. Co-extinctions of tropical butterflies and their hostplants. Biotropica. 2004;36:272–274. [Google Scholar]
- Kopp K., Jokela J. Resistant invaders can convey benefits to native species. Oikos. 2007;116:295–301. [Google Scholar]
- Kuris A.M., Hechinger R.F., Shaw J.C., Whitney K.L., Aguirre-Macedo L., Boch C.A., Dobson A.P., Dunham E.J., Fredensborg B.L., Huspeni T.C., Lorda J., Mababa1 L., Mancini F.T., Mora A.B., Pickering M., Talhouk N.L., Torchin M.E., Lafferty K.D. Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature. 2008;454:515–518. doi: 10.1038/nature06970. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Dobson A.P., Kuris A.M. Parasites dominate food web links. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K.D., Allesina S., Arim M., Briggs C.J., De Leo G., Dobson A.P., Dunne J.A., Johnson P.T.J., Kuris A.M., Marcogliese D.J., Martinez N.D., Memmott J., Marquet P.A., McLaughlin J.P., Mordecai E.A., Pascual M., Poulin R., Thieltges D.W. Parasites in food webs: the ultimate missing links. Ecol. Lett. 2008;11:533–546. doi: 10.1111/j.1461-0248.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K.D. Biodiversity loss decreases parasite diversity: theory and patterns. Philos. Trans. R. Soc. B. 2012;367:2814–2827. doi: 10.1098/rstb.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenski R.E., Ofria C., Pennock R.T., Adami C. The evolutionary origin of complex features. Nature. 2003;423:139–144. doi: 10.1038/nature01568. [DOI] [PubMed] [Google Scholar]
- Levi T., Kilpatrick A.M., Mangel M., Wilmers C.C. Deer, predators, and the emergence of lyme disease. Proc. Natl. Acad. Sci. U. S. A. 2012;109:10942–10947. doi: 10.1073/pnas.1204536109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T.J., Shuker D.M., Colegrave N., Day T., Graham A.L. The coevolution of virulence: tolerance in perspective. PLoS Pathog. 2010;6:e1001006. doi: 10.1371/journal.ppat.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcogliese D.J., Cone D.K. Parasite communities as indicators of ecosystem stress. Parassitologia. 1997;39:227–232. [PubMed] [Google Scholar]
- Marcogliese D.J. Parasites of the superorganism: are they indicators of ecosystem health? Int. J. Parasitol. 2005;35:705–716. doi: 10.1016/j.ijpara.2005.01.015. [DOI] [PubMed] [Google Scholar]
- McQuaid C.F., Britton N.F. Host–parasite nestedness: a result of co-evolving trait-values. Ecol. Complex. 2013;13:53–59. [Google Scholar]
- McQuaid C.F., Britton N.F. Coevolution of resource trade-offs driving species interactions in a host–parasite network: an exploratory model. Theor. Ecol. 2013;6:443–456. [Google Scholar]
- Mihaljevic J.R. Linking metacommunity theory and symbiont evolutionary ecology. Trends Ecol. Evol. 2012;27:323–329. doi: 10.1016/j.tree.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Moir M.L., Vesk P.A., Brennan K.E., Keith D.A., Hughes L., McCarthy M.A. Current constraints and future directions in estimating coextinction. Conserv. Biol. 2010;24:682–690. doi: 10.1111/j.1523-1739.2009.01398.x. [DOI] [PubMed] [Google Scholar]
- Mooney H.A., Cleland E.E. The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N.A. The evolution of host-plant alternation in aphids: evidence for specialization as a dead end. Am. Nat. 1988;132:681–706. [Google Scholar]
- Mu J., Joy D.A., Duan J., Huang Y., Carlton J., Walker J., Barnwell J., Beerli P., Charleston M.A., Pybus O.G., Su X.Z. Host switch leads to emergence of Plasmodium vivax malaria in humans. Mol. Biol. Evol. 2005;22:1686–1693. doi: 10.1093/molbev/msi160. [DOI] [PubMed] [Google Scholar]
- Nichols E., Gardner T.A., Peres C.A., Spector S. Co-declining mammals and dung beetles: an impending ecological cascade. Oikos. 2009;118:481–487. [Google Scholar]
- Ofria C., Wilke C.O. Avida: a software platform for research in computational evolutionary biology. Artif. Life. 2004;10:191–229. doi: 10.1162/106454604773563612. [DOI] [PubMed] [Google Scholar]
- Owens I.P., Bennett P.M. Ecological basis of extinction risk in birds: habitat loss versus human persecution and introduced predators. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12144–12148. doi: 10.1073/pnas.200223397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S.E., Cattadori I.M., Tagliapietra V., Rizzoli A.P., Hudson P.J. Localized deer absence leads to tick amplification. Ecology. 2006;87:1981–1986. doi: 10.1890/0012-9658(2006)87[1981:ldaltt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Perlman S.J., Jaenike J. Infection success in novel hosts: an experimental and phylogenetic study of Drosophila-parasitic nematodes. Evolution. 2003;57:544–557. doi: 10.1111/j.0014-3820.2003.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Poulin R. Richness, nestedness, and randomness in parasite infracommunity structure. Oecologia. 1996;105:545–551. doi: 10.1007/BF00330018. [DOI] [PubMed] [Google Scholar]
- Poulin R., Guégan J.F. Nestedness, anti-nestedness, and the relationship between prevalence and intensity in ectoparasite assemblages of marine fish: a spatial model of species coexistence. Int. J. Parasitol. 2000;30:1147–1152. doi: 10.1016/s0020-7519(00)00102-8. [DOI] [PubMed] [Google Scholar]
- Poulin R., Morand S. The diversity of parasites. Q. Rev. Biol. 2000;75:277–293. doi: 10.1086/393500. [DOI] [PubMed] [Google Scholar]
- Poulin R., Morand S. Smithsonian Institution Press; Washington, DC: 2004. Parasite Biodiversity. [Google Scholar]
- Poulin R. Parasite biodiversity revisited: frontiers and constraints. Int. J. Parasitol. 2014;449:581–589. doi: 10.1016/j.ijpara.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Prenter J., MacNeil C., Dick J.T., Dunn A.M. Roles of parasites in animal invasions. Trends Ecol. Evol. 2004;19:385–390. doi: 10.1016/j.tree.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Price R.D., Clayton D.H., Adams R.J. Pigeon lice down under: taxonomy of Australian Campanulotes (Phthiraptera: Philopteridae), with a description of C. durdeni n. sp. J. Parasitol. 2000;86:948–950. doi: 10.1645/0022-3395(2000)086[0948:PLDUTO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Raffel T.R., Martin L.B., Rohr J.R. Parasites as predators: unifying natural enemy ecology. Trends Ecol. Evol. 2008;23:610–618. doi: 10.1016/j.tree.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Rezende E.L., Lavabre J.E., Guimarães P.R., Jordano P., Bascompte J. Non-random coextinctions in phylogenetically structured mutualistic networks. Nature. 2007;448:925–928. doi: 10.1038/nature05956. [DOI] [PubMed] [Google Scholar]
- Rohde K., Worthen W.B., Heap M., Hugueny B., Guégan J.F. Nestedness in assemblages of metazoan ecto-and endoparasites of marine fish. Int. J. Parasitol. 1998;28:543–549. doi: 10.1016/s0020-7519(98)00013-7. [DOI] [PubMed] [Google Scholar]
- Roopnarine P.D. Extinction cascades and catastrophe in ancient food webs. Paleobiology. 2006;32:1–19. [Google Scholar]
- Sahasrabudhe S., Motter A.E. Rescuing ecosystems from extinction cascades through compensatory perturbations. Nat. Commun. 2011;2:170. doi: 10.1038/ncomms1163. [DOI] [PubMed] [Google Scholar]
- Schmidt K.A., Ostfeld R.S. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Sih A. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 2013;85:1077–1088. [Google Scholar]
- Sokolow S.H., Huttinger E., Jouanard N., Hsieh M.H., Lafferty K.D., Kuris A.M., Riveau G., Senghor S., Thiam C., N'Diaye A., Sarr Faye D., De Leo G.A. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc. Natl. Acad. Sci. U. S. A. 2015;112:9650–9655. doi: 10.1073/pnas.1502651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan D.P. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork N.E., Lyal C.H. Extinction or ‘co-extinction’ rates? Nature. 1993;366:307. [Google Scholar]
- Stringer A.P., Linklater W. Everything in moderation: principles of parasite control for wildlife conservation. BioScience. 2014;64:932–937. [Google Scholar]
- Strona G., Lafferty K.D. FishPEST: an innovative software suite for fish parasitologists. Trends Parasitol. 2012;28:123. doi: 10.1016/j.pt.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Strona G., Lafferty K.D. Predicting what helminth parasites a fish species should have using parasite co-occurrence modeler (PaCo) J. Parasitol. 2013;99:6–10. doi: 10.1645/GE-3147.1. [DOI] [PubMed] [Google Scholar]
- Strona G., Galli P., Fattorini S. Fish parasites resolve the paradox of missing coextinctions. Nat. Commun. 2013;4:1718. doi: 10.1038/ncomms2723. [DOI] [PubMed] [Google Scholar]
- Strona G. Assessing fish vulnerability: IUCN vs FishBase. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014;24:153–154. [Google Scholar]
- Strona G., Fattorini S. Parasitic worms: how many really? Int. J. Parasitol. 2014;44:269–272. doi: 10.1016/j.ijpara.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Strona G., Fattorini S. A few good reasons why species-area relationships do not work for parasites. BioMed Res. Int. 2014:271680. doi: 10.1155/2014/271680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strona G., Fattorini S. On the methods to assess significance in nestedness analyses. Theory Biosci. 2014;133:179–186. doi: 10.1007/s12064-014-0203-1. [DOI] [PubMed] [Google Scholar]
- Strona G. The underrated importance of predation in transmission ecology of direct lifecycle parasites. Oikos. 2015;124:685–690. [Google Scholar]
- Strona G., Veech J.A. A new measure of ecological network structure based on node overlap and segregation. Methods Ecol. Evol. 2015;6:907–915. [Google Scholar]
- Strona G., Fattorini S. Are generalist parasites being lost from their hosts? J. Anim. Ecol. 2015 doi: 10.1111/1365-2656.12443. [DOI] [PubMed] [Google Scholar]
- Telfer S., Lambin X., Birtles R., Beldomenico P., Burthe S., Paterson S., Begon M. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.D., Cameron A., Green R.E., Bakkenes M., Beaumont L.J., Collingham Y.C., Erasmus B.F.N., Ferreira de Siqueira M., Grainger A., Hannah L., Hughes L., Huntley B., van Jaarsveld A.S., Midgley G.F., Miles L., Ortega-Huerta M.A., Peterson A.T., Phillips O.L., Williams S.E. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Torchin M.E., Lafferty K.D., Dobson A.P., McKenzie V.J., Kuris A.M. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- Torchin M.E., Mitchell C.E. Parasites, pathogens, and invasions by plants and animals. Front. Ecol. Environ. 2004;2:183–190. [Google Scholar]
- Traill L.W., Lim M.L., Sodhi N.S., Bradshaw C.J. Mechanisms driving change: altered species interactions and ecosystem function through global warming. J. Anim. Ecol. 2010;79:937–947. doi: 10.1111/j.1365-2656.2010.01695.x. [DOI] [PubMed] [Google Scholar]
- Vázquez D.P., Poulin R., Krasnov B.R., Shenbrot G.I. Species abundance and the distribution of specialization in host–parasite interaction networks. J. Anim. Ecol. 2005;74:946–955. [Google Scholar]
- Vázquez D.P., Melián C.J., Williams N.M., Blüthgen N., Krasnov B.R., Poulin R. Species abundance and asymmetric interaction strength in ecological networks. Oikos. 2007;116:1120–1127. [Google Scholar]
- Veech J.A. A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 2013;22:252–260. [Google Scholar]
- Walther G.R., Post E., Convey P., Menzel A., Parmesan C., Beebee T.J., Fromentin J.M., Hoegh-Guldberg O., Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Wake D.B., Vredenburg V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J.V., Elliott D.E. Helminths and the IBD hygiene hypothesis. Inflamm. Bowel Dis. 2009;15:128–133. doi: 10.1002/ibd.20633. [DOI] [PubMed] [Google Scholar]
- Whiteman N.K., Parker P.G. Using parasites to infer host population history: a new rationale for parasite conservation. Anim. Conserv. 2005;8:175–181. [Google Scholar]
- Whitlock M.C. The red queen beats the jack-of-all-trades: the limitations on the evolution of phenotypic plasticity and niche breadth. Am. Nat. 1996;148:S65–S77. [Google Scholar]
- Wiegmann B.M., Mitter C., Farrell B. Diversification of carnivorous parasitic insects: extraordinary radiation or specialized dead end? Am. Nat. 1993;142:737–754. [Google Scholar]
- Windsor D.A. Heavenly hosts. Nature. 1990;348:104. [Google Scholar]
- Windsor D.A. Equal rights for parasites. Conserv. Biol. 1995;9:1–2. doi: 10.1353/pbm.1997.0011. [DOI] [PubMed] [Google Scholar]
- Windsor D.A. Equal rights for parasites. Perspect. Biol. Med. 1997;40:222–229. doi: 10.1353/pbm.1997.0011. [DOI] [PubMed] [Google Scholar]
- Windsor D.A. Stand up for parasites. Trends Ecol. Evol. 1997;12:32. doi: 10.1016/s0169-5347(97)88390-5. [DOI] [PubMed] [Google Scholar]
- Windsor D.A. Controversies in parasitology, most of the species on earth are parasites. Int. J. Parasitol. 1998;28:1939–1941. doi: 10.1016/s0020-7519(98)00153-2. [DOI] [PubMed] [Google Scholar]
- Wood J.R., Wilmshurst J.M., Rawlence N.J., Bonner K.I., Worthy T.H., Kinsella J.M., Cooper A. A Megafauna's microfauna: gastrointestinal parasites of New Zealand's extinct moa (aves: Dinornithiformes) PLoS ONE. 2013;8:e57315. doi: 10.1371/journal.pone.0057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh M., Kremsner P.G., van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- Zaman L., Devangam S., Ofria C. Proceedings of the 13th Annual Conference on Genetic and Evolutionary Computation. ACM; 2011. Rapid host-parasite coevolution drives the production and maintenance of diversity in digital organisms; pp. 219–226. [Google Scholar]
- Zaman L., Meyer J.R., Devangam S., Bryson D.M., Lenski R.E., Ofria C. Coevolution drives the emergence of complex traits and promotes evolvability. PLoS Biol. 2014;12:e1002023. doi: 10.1371/journal.pbio.1002023. [DOI] [PMC free article] [PubMed] [Google Scholar]