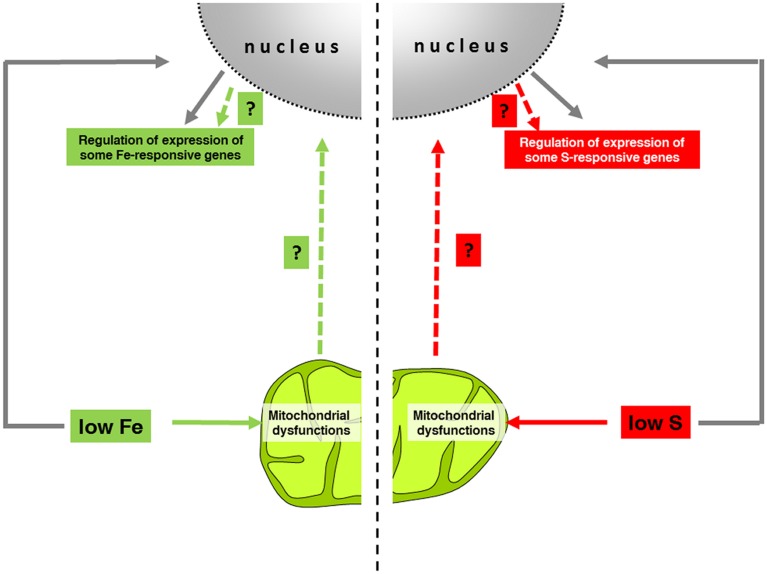
Figure 1.
Schematized model of the possible role of mitochondria in the regulation of nutrient-responsive genes. As Fe and S are essential elements for Fe–S cluster-containing proteins, deficiencies of such nutrients trigger mitochondrial dysfunctions mainly at the respiratory chain level. Through the comparison of different data sets (mitochondrial dysfunctions vs. Fe- and/or S-responsive genes), nutrient-responsive genes have been identified as potential candidates of hypothetical retrograde signaling pathways (PYE, BTS, FRO3; OPT3, SAM1 for Fe homeostasis and SULTR1;2 SULTR3;4, SULTR4;2, LSU1, BGLU28, SDI1 for S homeostasis). Mitochondrial impairments occur in long term of Fe and S starvations; therefore, such retrograde signaling would occur in a second phase of the regulation of nuclear gene expression, whereas the first phase (gray arrows) involved a more direct nutrient-sensing mechanisms.