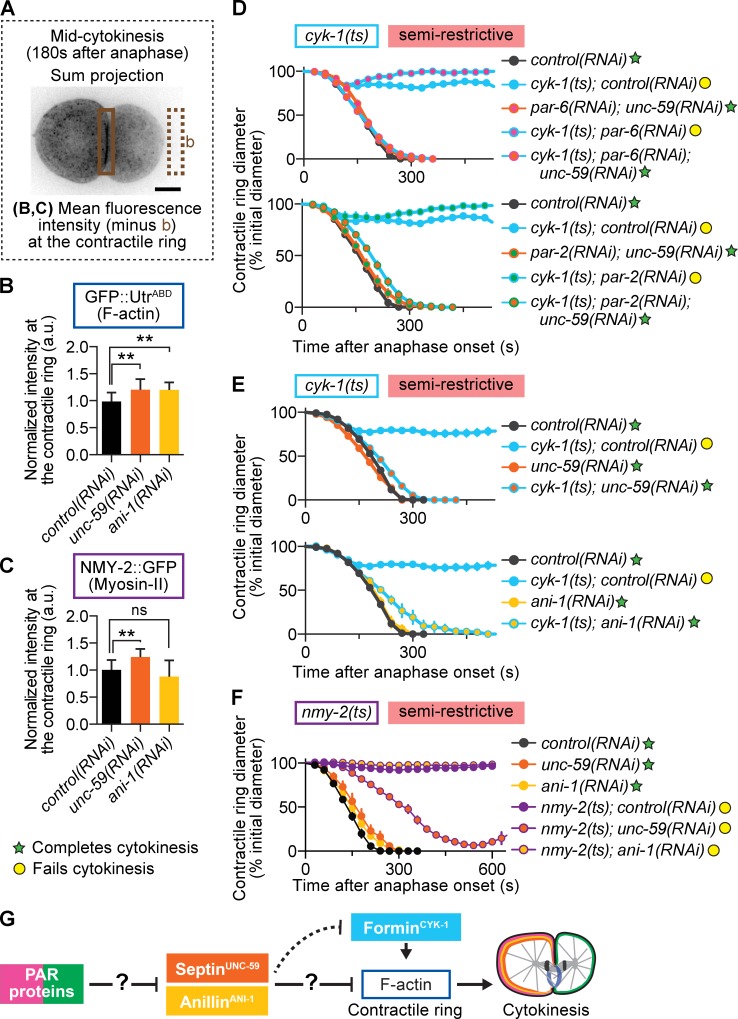
Figure 5.
Septin and anillin restrict contractile ring F-actin levels and negatively regulate cytokinesis. (A) Schematic of region and formula used for analysis shown in B and C. The boxed area represents the region used for measurement in the contractile ring. (B and C) Depletion of septin and anillin increased F-actin levels in the contractile ring (B), whereas only septin depletion increased myosin-II levels in the ring (C; n ≥ 10, all conditions; mean ± SD). **, P < 0.005; ns, not significant. (D) Depletion of septin suppresses cytokinesis failure in formin(ts) mutant embryos at semirestrictive temperature, even when A-P polarity is simultaneously disrupted (control(RNAi) n = 9, cyk-1(ts);control(RNAi) n = 11, par-6(RNAi);unc-59(RNAi) n = 11, cyk-1(ts);par-6(RNAi) n = 3, cyk-1(ts);par-6(RNAi);unc-59(RNAi) n = 12, par-2(RNAi);unc-59(RNAi) n = 10, cyk-1(ts);par-2(RNAi) n = 4, cyk-1(ts);par-2(RNAi);unc-59(RNAi) n = 12; mean ± SEM). (E) Depletion of septin and anillin suppresses cytokinesis failure in formin(ts) mutant embryos at semirestrictive temperature (n = 10, all conditions; mean ± SEM). (F) RNAi-mediated depletion of neither anillin nor septin suppresses cytokinesis failure in myosin-II(ts) mutant embryos at semirestrictive temperature (n ≥ 8, all conditions; mean ± SEM). (G) In our genetic model, PAR proteins inhibit septin and anillin localization in the contractile ring by promoting their anterior retention. This prevents septin and anillin from inhibiting F-actin and thereby promotes robust cytokinesis. The question marks reflect that the mechanism of action, whether via a direct interaction or other intermediate players in the pathway, is unknown. Bar, 10 µm. a.u., arbitrary units; b, background. cyk-1 = formin; nmy-2 = myosin-II; ani-1 = anillin; unc-59 = septin.