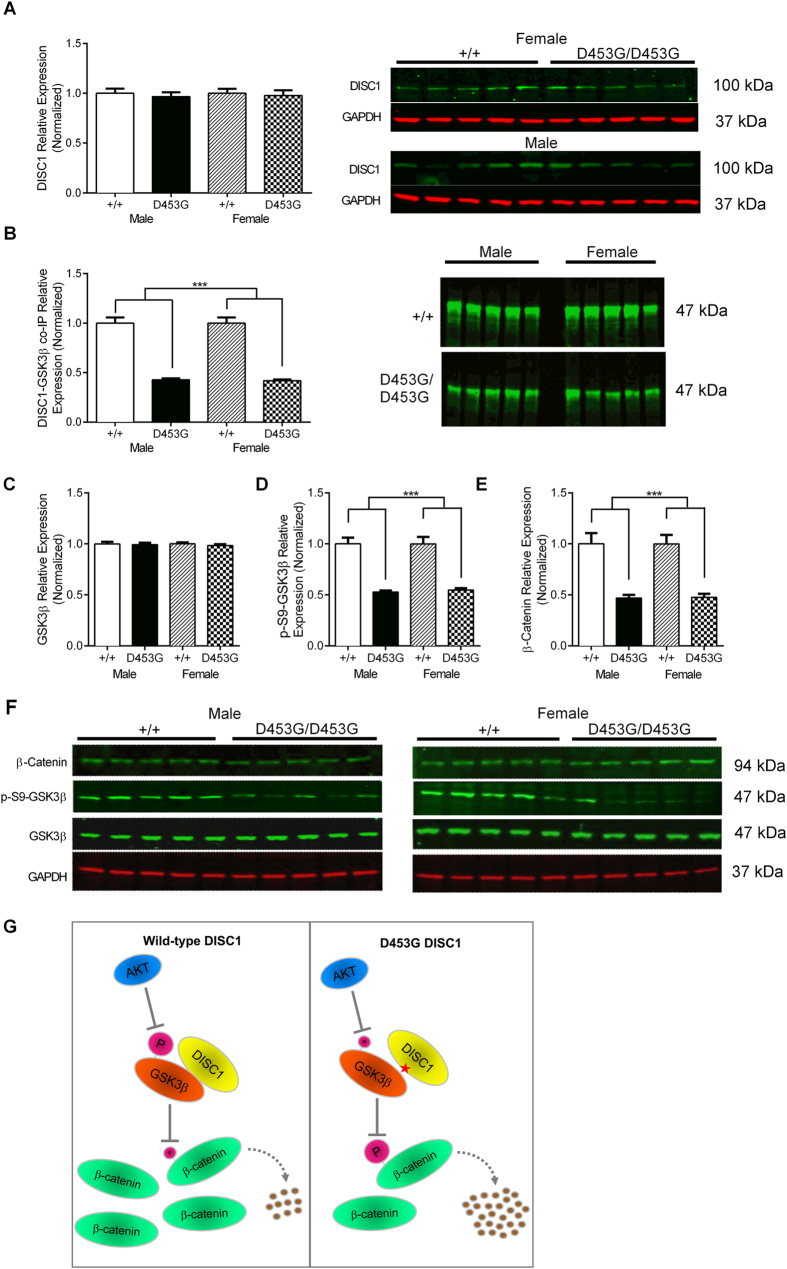
Figure 6. DISC1D453G disrupts GSK3β signaling.
(A) Western blotting revealed comparable levels of DISC1 protein in whole brain homogenates from wild-type (+/+; n = 10, 5 male and 5 female) and DISC1D453G (D453G/D453G; n = 10, 5 male and 5 female) mice (left side; mean of three gels). Representative blots of DISC1 and GAPDH loading control for male and female DISC1D453G mice (right side). (B) The ability of DISC1 to complex with GSK3β was tested by co-immunoprecipitation (co-IP). DISC1D453G significantly reduced DISC1-GSK3β binding in both male and female DISC1D453G mice. (C) GSK3β protein levels were comparable between wild-type and DISC1D453G mice of either sex. (D) Levels of S9 phosphorylated GSK3β (p-S9-GSK3β) normalized to total GSK3β were significantly decreased in whole brain homogenates from both male and female DISC1D453G mice. (E) β-catenin protein levels were decreased in whole brain homogenates from DISC1D453G mice of either sex. (F) Representative blots for male (left side) and female (right side) wild-type and DISC1D453G mice. (G) Schematic model depicting the effects of D453G on GSK3β signaling. Mutation D453G reduces the interaction of DISC1 with GSK3β, and AKT-dependent phospho-inhibition of GSK3β at S9 is decreased. The less inhibited GSK3β phosphorylates more β-catenin, leading to more ubiquitin-mediated degradation of β-catenin, and reducing the cellular level of β-catenin. Red star: D453G mutation; T-shaped arrows: inhibition; magenta circles: phosphate group, size indicating degree of phospho-inhibition; curved broken arrow: ubiquitin-mediated degradation; brown circles: products of β-catenin proteolysis.