Abstract
The objective of this study was to compare the effects of adding lactic acid and pectinase, and chaptalization for the quality of apple wine and the production of hazardous compounds (methanol and acetaldehyde). The pH of all of the samples was below 4; therefore, mash seemed to be fermented without any issue. Total acidity was the highest in sample A due to lactic acid addition. Pre-treated groups (samples B, C, and D) showed higher total acidities than that of the control (P<0.05). Pre-treatments might influence the production of organic acids in apple wines. The control and pectinase added sample (sample B) had the lowest alcohol contents. Adding lactic acid produced more alcohol, and chaptalized samples produced more alcohol due to the addition of sugar. Adding pectinase with and without chaptalization was not effective for producing more alcohol. The control sample had significantly higher acetaldehyde content (2.39 mg/L) than the other samples (1.00~2.07 mg/L); therefore, pre-treatments for apple wine fermentation produced a lower amount of acetaldehyde. Among the pre-treated samples, samples C and D showed the lowest acetaldehyde content of 1.00 mg/L and 1.16 mg/L, respectively. On the other hand, a significantly higher amount of methanol was generated for sample A (1.03 mg/L) and sample D (1.22 mg/L) than that of the control (0.82 mg/L) (P<0.05). Adding lactic acid or chaptalization was effective in reducing methanol and acetaldehyde in apple wines.
Keywords: apple, wine, pre-treatment, fermentation, acetaldehyde
INTRODUCTION
The apple (Malus domestica “Fuji”) is a fruit that is grown and consumed consistently in Korea, and it contains aboundant minerals, vitamins, and fibers (1). The apple has various functional compounds including polyphenol compounds, and it also shows antioxidant activity (2). The apple is consumed mainly as a fruit, and processed as juice, jam, snacks, and vinegar (3). The apple cultivation area in Korea has not increased since 2008, which is over 30,000 ha (4). In 2014, the apple cultivation area was 30,702 ha, and this shows that apple consumption is has not increasing recently. Processed foods from apples are necessary to increase apple productions and income for farmers because apples are high valued agricultural products.
Alcoholic beverages, especially spirits, are produced from agricultural products such as grape, barley, corn, and potatoes. They create more value being sold as alcoholic beverages than being sold as raw ingredients. The liquor business can create more value than other processed foods such as juice, jam, or canned fruits. The apple is one of main fruits in Korea; however, studies on alcoholic beverages are limited in Korea. Apple wine studies were first introduced in the 1970s and 1980s. Reports on the influence of maceration in apple wine brewing (5) and polyphenol compounds during apple wine fermentation (6) were reported. Chung et al. (7) studied applying ultrafiltration for processing of apple wine. Recently, brewing “ice apple wine” using Saccharomyces cerevisiae SS89 (8), which has a glucose tolerance characteristic, and quality characteristics of apple wines with medicinal herbs (9) were reported. Various processing conditions such as pre-treatment, the fermentation process, and temperature are necessary to produce high quality apple alcoholic beverages (10–12).
In order to brew alcoholic beverages using fruits, food additives and additional ingredients are added for safety and/or processing issues. Sulfurous acids are added to prevent oxidation of fruit juice and growth of hazardous microorganisms during fermentation although they can generate allergies (8,13–15). Lactic acid is added to drop the pH of mash in order to prevent the growth of unrelated microorganisms for alcohol fermentation (16). A pH lower than 3.5 for apple wine is known as an appropriate pH for apple wine fermentation (17,18). Pectinase is added to fruit wine mash to make the juice transparent and to increase the amount of juice (19). However, using pectinase could generate methanol during fermentation, so special care is necessary (20). Since the soluble solid of fruit or fruit juice for wine production is not high enough to make a sufficient amount of alcohol, adding sugar at the beginning of fermentation, which is known as chaptalization, is frequently conducted to produce more alcohol (21). Sugar in the mash can be used as an energy source for yeasts and prevent heterofermentation that happens from the lack of an energy source (22). The effects of these pre-treatments for apple wine mash have not been investigated. Therefore, the objective of this study was to compare the effects of adding lactic acid and pectinase, and chaptalization for the quality of apple wine and the production of hazardous compounds (methanol and acetaldehyde).
MATERIALS AND METHODS
Apple wine mash
Apples (Malus domestica “Fuji”) were purchased at a single apple orchard harvested in 2013 (Anseong, Korea). Apples were washed twice using tap water and drained 1 h to remove water from the surface of the apples. They were diced to 0.7×0.7 cm pieces after removing the skin and ovary of apples using a dicer (SL Dicer MCD-380M, UpsoKorea, Goyang, Korea). The diced apples (10 kg) were crushed by a food processor and then put into a fermenter (20 L). K2S2O5 (100 mg/kg of diced apple) was added to the fermenter and kept for 5 h (23). Yeast (20 g; Fermivin, DSM, Heerlen, Denmark), widely used for wine fermentation, was added to the fermenters and stirred. Additional ingredients for pre-treatments of the mash are shown in Table 1. Lactic acid (1 mL/kg of diced apple) was added to sample A to lower the pH at the initial stage of fermentation. Pectinase (100 mg/kg of diced apple) was added to sample B in order to make the wine clear and generate more energy source for yeasts. Chaptalization to 24°Brix was conducted for sample C to produce more alcohol by adding 1,500 g of sugar. Pectinase (100 mg/kg of diced apple) and chaptalization (1,500 g of sugar) were carried out for sample D. Fermenters were put in a water bath and maintained at fermentation temperature at 20°C. Fermentation was conducted for 9 days. Fermenters were stirred every 12 h for uniform fermentation.
Table 1.
Pre-treatment conditions of mash for apple wine fermentation
| Sample | Treatment | |||
|---|---|---|---|---|
|
| ||||
| K2S2O5 (mg/kg apple) | Lactic acid (mL/kg apple) | Pectinase (mg/kg apple) | Chaptalization (°Brix) | |
| Control | 100 | – | – | – |
| A | 100 | 1.0 | – | – |
| B | 100 | – | 100 | – |
| C | 100 | – | – | 24 |
| D | 100 | – | 100 | 24 |
Physicochemical characteristics of fermented mash
The following measurements that showed the fermentation characteristics during 9 days were conducted by the Liquors License Aid Center in the Korean National Tax Services (24). Apple wine mash was filtered using a paper filter (pore size: 5 μm, Hyundai Micro Co., Ltd., Seoul, Korea) to remove solid parts. The results were measured at 23°C. Alcohol contents were converted to the alcohol contents at 15°C. All measurements were triplicated.
pH and total acidity
A pH meter (ST3000, OHAUS Co., Parsippany, NJ, USA) was used for the pH measurements of the mash. A sample (10 mL) was titrated with a 0.1 N NaOH solution until the pH meter indicated 7.00±0.05. The amount of 0.1 N NaOH solution was converted to 0.05% acetic acid equivalent.
Total soluble content
Filtered mash (0.5 mL) was put on a digital refractometer (HI 96801, Hanna Instruments, Woonsocket, RI, USA) to measure soluble solid contents.
Alcohol content
Alcohol content was measured using an automated electronic density meter (DMA4100M, Anton Paar GmbH, Graz, Austria).
Analysis of acetaldehyde and methanol contents
The analyses of acetaldehyde and methanol were conducted with slight modification of the methods by Kim et al. (25) and Lee et al. (26). The amounts of acetaldehyde and methanol of apple wines were quantified by gas-chromatography (GC; Agilent Technologies, Santa Clara, CA, USA). Filtered apple wine passed through a 0.45 μm membrane filter and then a 0.22 μm membrane filter. A sample (1 μL) was injected into a GC. N2 gas (1 mL/min) was used as a carrier gas. DB-WAX column (i.d. 0.25 mm×30 m, film 0.25 μm; Restek Co., Bellefonte, PA, USA) was used for the analyses. Temperature settings were the following: injector and detector temperature: 200°C; oven temperature: 45°C for 1 min, increasing 7°C/min to 130°C and maintained 1 min. A flame ionization detector was used for acetaldehyde and methanol detection. The peak areas of the chromatogram of acetaldehyde and methanol were compared to the peak areas of standard acetaldehyde (≥99.5% for GC, Sigma-Aldrich, St. Louis, MO, USA) and methanol (≥99.8% for GC, Sigma-Aldrich) for quantification. Experiments were triplicated and the mean values were used for calculation.
Statistical analyses
One-way analysis of variance (ANOVA) was conducted at P<0.05 using XLSTAT version 2012 (Addinsoft, Paris, France). When statistical significances were found by ANOVA, Fisher’s least significant difference test was performed at P<0.05.
RESULTS AND DISCUSSION
Physicochemical characteristics
The pH of apple wines during 9 days of fermentation is shown in Table 2. These generally showed small variations during fermentation and important parameters for the identification of heterofermentation (27,28). A pH lower than 4 is considered as homofermentation without contamination (29); therefore, all samples seemed to be safely fermented by presenting a pH lower than 4 in this study. The control and sample C showed the highest pH during the fermentation period. Chaptalization (sample C) did not influence a lowering of the pH of the mash. Sample A showed the lowest pH at day 1 because lactic acid was added to the mash and then gradually increased to pH 3.42. Since there was more lactic acid in the mash in sample A, lactic acid bacteria could easily proliferate compared to the other samples (30). Adding pectinase also significantly dropped the pH of the mash in comparison with the control (P<0.05, results not shown) and could lower the chance of being heterofermented (P<0.05). The pH patterns of each sample were similar to grape wine fermentation in that they showed low variations during the 9 days of fermentation, except sample A because it contained lactic acid (31). However, the pH patterns of apple wine mash were different from Makgeolli, which made sugars from the degradation of carbohydrates by enzymes (32).
Table 2.
pH change of apple wine mash during nine days of fermentation
| Sample | pH | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | |
| Control | 3.97±0.04a1) | 3.81±0.01a | 3.82±0.02a | 3.80±0.03a | 3.80±0.01a | 3.83±0.01a | 3.85±0.01a | 3.89±0.03a | 3.91±0.02a |
| A | 3.14±0.03c | 3.07±0.03c | 3.09±0.02d | 3.12±0.02c | 3.19±0.02c | 3.26±0.02c | 3.29±0.03c | 3.38±0.02c | 3.42±0.01d |
| B | 3.63±0.03b | 3.49±0.03b | 3.47±0.04c | 3.48±0.04b | 3.49±0.03b | 3.52±0.02b | 3.56±0.02b | 3.60±0.02b | 3.61±0.03c |
| C | 3.90±0.10a | 3.80±0.08a | 3.80±0.03a | 3.81±0.06a | 3.80±0.06a | 3.81±0.06a | 3.83±0.04a | 3.84±0.05a | 3.84±0.05b |
| D | 3.69±0.03b | 3.53±0.02b | 3.52±0.02b | 3.52±0.03b | 3.51±0.03b | 3.53±0.04b | 3.55±0.03b | 3.59±0.03b | 3.59±0.02c |
Different letters (a–d) in the same column indicate significant difference at P<0.05 by Fisher’s least significant difference test.
Total acidities of apple wine during the 9 days of fermentation are presented in Table 3. Total acidity gradually increased from day 1 to day 9. The total acidity of the control was 7.37% at the end of the fermentation period. Adding lactic acid (sample A) significantly influenced the total acidity and the amount of lactic acid was the main reason for the highest total acidity. The generation of organic acid by the yeasts was lower (0.94%) than those of the other samples (2.76~3.94%) when the total acidity of day 1 was subtracted from that of day 9. Total acidity of sample B (8.63%) was significantly higher than that of the control (7.37%) at the end of the fermentation period. Adding pectinase could be effective in producing more organic acid by generating sugars for yeast fermentation (33). Total acidity by chaptalization (8.20%; sample C) produced more organic acid than the control (7.37%). This is due to the yeasts that used sugar in making organic acid during the fermentation period (34). Sample D was treated by adding pectinase and chaptalizing, and these two pre-treatments in the mash showed a synergistic effect by showing 9.47% of total acidity. The increasing total acidity during fermentation was the same as grape wine (31) and Makgeolli added to kiwifruit (35) fermentation.
Table 3.
Total acidity of apple wine mash during nine days of fermentation
| Sample | Total acidity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | |
| Control | 4.33±0.12c1) | 5.00±0.20c | 5.90±0.10d | 6.43±0.06d | 6.67±0.12c | 7.03±0.15e | 7.20±0.10e | 7.43±0.15e | 7.37±0.12e |
| A | 13.13±0.23a | 13.03±0.21a | 12.80±0.20a | 12.33±0.31a | 12.20±0.20a | 12.73±0.12a | 13.43±0.06a | 13.93±0.12a | 14.07±0.15a |
| B | 5.87±0.31b | 6.53±0.15b | 7.40±0.20c | 8.17±0.15c | 8.27±0.31b | 8.37±0.15c | 8.53±0.12c | 8.53±0.15c | 8.63±0.15c |
| C | 4.40±0.30c | 5.30±0.46c | 5.87±0.06d | 6.63±0.12d | 6.90±0.36c | 7.37±0.06d | 7.67±0.21d | 7.93±0.42d | 8.20±0.35d |
| D | 5.53±0.12b | 6.23±0.15b | 7.77±0.15b | 8.63±0.25b | 8.40±0.44b | 8.83±0.06b | 9.20±0.20b | 9.47±0.23b | 9.47±0.21b |
Different letters (a–e) in the same column indicate significant difference at P<0.05 by Fisher’s least significant difference test.
Total soluble solid contents during the fermentation period are presented in Table 4. As fermentation continued, total soluble solid contents decreased. Yeasts used a sugar source to produce alcohols (36). The control used 6.43°Brix for fermentation. Non-chaptalized samples (samples A and B) used 7.80 and 5.83°Brix for alcohol production, respectively. Chaptalized samples used 12.20 and 10.87°Brix during fermentation. Adding lactic acid (sample A) caused a significant drop between day 3 and 5. Adding pectinase (Sample B) generated more total soluble solid content after fermentation than that of the control. Pectinase breaks pectin into oligo- and poly-saccharides and increases the total soluble solid content (37). There was no significant difference in the addition of pectinase when chaptalized for fermentation.
Table 4.
Changes in total soluble solid content during nine days of apple wine fermentation
| Sample | Total soluble solid content (°Brix) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | |
| Control | 12.00±0.20c1) | 9.47±0.06d | 7.67±0.15e | 6.53±0.12a | 5.87±0.15e | 5.67±0.12d | 5.53±0.15d | 5.53±0.06c | 5.57±0.06c |
| A | 14.03±0.21b | 14.20±0.17b | 12.37±0.15c | 9.47±0.31c | 7.60±0.20c | 7.27±0.31b | 6.63±0.32c | 6.43±0.40b | 6.23±0.21b |
| B | 12.23±0.21c | 10.23±0.25c | 8.80±0.20d | 7.40±0.20d | 6.67±0.12d | 6.50±0.10c | 6.43±0.25c | 6.37±0.15b | 6.40±0.10b |
| C | 23.83±0.21a | 19.30±0.10a | 17.63±0.45b | 16.23±0.21b | 15.17±0.45b | 14.07±0.42a | 13.17±0.35b | 12.80±0.40a | 12.63±0.45a |
| D | 23.80±0.20a | 19.40±0.20a | 18.10±0.10a | 17.10±0.46a | 15.87±0.23a | 14.47±0.42a | 13.87±0.42a | 13.27±0.50a | 12.93±0.42a |
Different letters (a–e) in the same column indicate significant difference at P<0.05 by Fisher’s least significant difference test.
Alcohol production
Alcohol contents of the sample during the 9 days of fermentation are presented in Table 5. The control produced 6.47% (v/v) of alcohol content after the 9 days of fermentation. Sample A showed no alcohol content until the second day of fermentation. This is due to a drop in pH by adding lactic acid (38). Fermentation started after 72 h due to the adaptation period for yeasts in low pH. Sample A had significantly higher alcohol content than the control at day 8 (P<0.05). Sample B, which had pectinase, showed similar alcohol production during 9 days of fermentation in comparison with the control. There was no significant increase in alcohol content in comparison with the control. This result was similar to the alcohol content of apple wine using the “Idared” variety (32). Alcohol content (8.60%, v/v) of a pectinolytic enzyme added to a sample did not produce significantly higher ethanol than the control (8.35%, v/v). Chaptalization (sample C) was effective for producing more alcohol since yeasts can use more of the energy source during fermentation (39). When alcohol content was divided into total soluble solid content, the alcohol content/total soluble solid ratio for the control and sample C, were 0.51 and 0.50, respectively. Therefore, the effectiveness of alcohol production by chaptalization was the same as the control. Whether to conduct chaptalization for apple wine brewing might be dependent on the cost of apples and sugar or the marketing point for breweries. The alcohol content by adding pectinase when chaptalized (11.70%, v/v; sample D) was not significantly different from sample C (11.97%, v/v). Adding pectinase in chaptalized apple wine mash was not effective in producing more alcohol.
Table 5.
Alcohol contents of apple wine mash during nine days of fermentation
| Sample | Alcohol content (%, v/v) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | |
| Control | 1.37±0.15a1) | 3.50±0.10b | 5.23±0.25b | 6.03±0.25b | 6.13±0.12c | 6.33±0.12b | 6.43±0.15b | 6.43±0.12c | 6.47±0.15c |
| A | 0.00±0.00b | 0.00±0.00c | 1.90±0.61d | 4.00±0.20c | 5.27±0.15d | 5.93±0.15b | 6.40±0.20b | 6.87±0.06b | 7.03±0.06b |
| B | 1.40±0.20a | 3.17±0.15b | 4.17±0.21c | 5.80±0.20b | 6.13±0.12c | 6.33±0.12b | 6.40±0.10b | 6.53±0.12bc | 6.53±0.06c |
| C | 1.20±0.17a | 4.10±0.26a | 6.07±0.12a | 8.07±0.12a | 9.53±0.50a | 10.10±0.46a | 11.00±0.30a | 11.83±0.45a | 11.97±0.35a |
| D | 1.27±0.06a | 3.30±0.26b | 6.17±0.21a | 8.00±0.20a | 8.60±0.20b | 9.73±0.12a | 10.67±0.35a | 11.60±0.20a | 11.70±0.36a |
Different letters (a–d) in the same column indicate significant difference at P<0.05 by Fisher’s least significant difference test.
Quantification of acetaldehyde and methanol
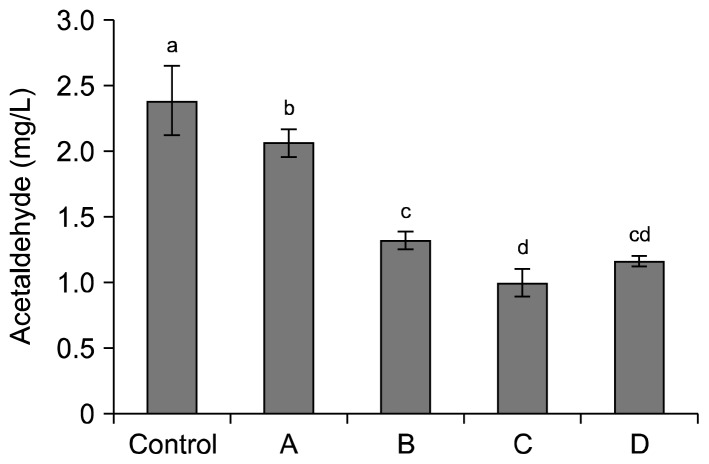
Acetaldehyde is produced by yeasts during alcohol fermentation and known as Group 2B carcinogen (40). The amounts of acetaldehyde in apple wines made with various pre-treatments of mash are shown in Fig. 1. The control had the highest content of acetaldehyde (2.39 mg/L). Sample A showed significantly lower acetaldehyde content (2.07 mg/L) than that of the control (P<0.05). The drop in pH by acing lactic acid seemed to be effective in lowering the acetaldehyde content of apple wine. Adding pectinase produced lower acetaldehyde (1.32 mg/L) than that of sample A. Production of sugar sources (mono- and oligosaccharides) by decomposing pectin, pectinic acid, and pectic acid could prevent heterofermentation by supplying adequate energy to yeasts during fermentation. This is in line with a higher soluble solid content in sample B than that in the control on the ninth day of fermentation (Table 5). Chaptalization to 24°Brix (sample C) was the most effective in reducing acetaldehyde synthesis in apple wine by presenting the lowest content (1.00 mg/L). Interestingly, sample D, which had pectinase and chaptalization, showed slightly higher acetaldehyde content than sample C. Adding pectinase alone reduced acetaldehyde content; however, it was not effective when chaptalization was performed. Yeasts used sugars from chaptalization as well as sugars produced by decomposing polysaccharides from apples. Since pure sugar could be directly used for the energy source of yeast, oligosaccharide produced by pectinase would prevent the use of sugar for some yeast. The competition between sugar and oligosaccharide generated a slightly higher amount of acetaldehyde than that of sample C.
Fig. 1.
Acetaldehyde content (mg/L) of apple wines fermented by different pre-treatment conditions of mash. Pre-treatment conditions referred to Table 1. Different letters (a–d) indicate significant difference at P<0.05 by Fisher’s least significant difference test.
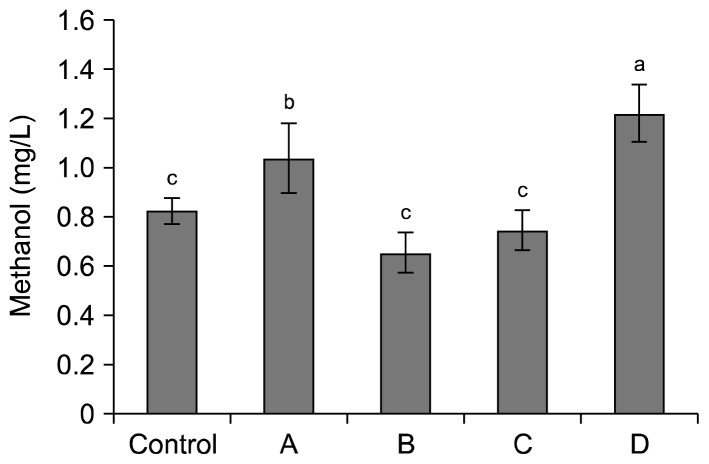
Methanol content of the samples is presented in Fig. 2. The amounts of methanol in these samples were within the range (0~769 mg/L) of apple wine (29). The control produced 0.82 mg/L of methanol. Dropping the pH by adding lactic acid (sample A) showed significantly higher methanol content (1.03 mg/L) than that of the control. This was due to the low pH of the mash (41). Methanol of samples B (0.65 mg/L) and C (0.74 mg/L) were statistically identical to the control (P<0.05), whereas adding pectinase and sugar (sample D) produced significantly higher methanol (1.22 mg/L) than those from adding pectinase (sample B) and adding sugar (sample C). It is expected that a synergistic effect in the production of methanol would be carried out for sample D.
Fig. 2.
Methanol content (mg/L) of apple wines fermented by different pre-treatment conditions of mash. Pre-treatment conditions referred to Table 1. Different letters (a–c) indicate significant difference at P<0.05 by Fisher’s least significant difference test.
In conclusion, adding lactic acid was effective in producing more alcohol than the control and reduced acetaldehyde content. Unless chaptalization is performed, adding lactic acid for apple wine mash would be beneficial in preventing contamination and producing more alcohol. Chaptalized samples produced more alcohol due to the additional energy source for alcohol fermentation. The additional alcohol by adding sugar had higher total acidity than the control with the same conversion ratio between alcohol content and total soluble solid content. If sugar is cheaper than apples, chaptalizing would be beneficial to apple wine breweries with a cost benefit.
ACKNOWLEDGEMENTS
The research was supported by the High Value-added Food Technology Development Program of the Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Hwang TY, Son SM, Lee CY, Moon KD. Quality changes of fresh-cut packaged Fuji apples during storage. Korean J Food Sci Technol. 2001;33:469–473. [Google Scholar]
- 2.Wojdyło A, Oszmiański J, Laskowski P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J Agric Food Chem. 2008;56:6520–6530. doi: 10.1021/jf800510j. [DOI] [PubMed] [Google Scholar]
- 3.Yun HJ, Lim SY, Hur JM, Jeong JW, Yang SH, Kim DH. Changes of functional compounds in, and texture characteristics of, apples during post-irradiation storage at different temperatures. Korean J Food Preserv. 2007;14:239–246. [Google Scholar]
- 4.KOSIS. Cultivation area of apple. Korean Statistical Information Service; Daejeon, Korea: 2014. [Google Scholar]
- 5.Chung KT, Song HI. Effects of maceration of fresh pulp on apple wine making. Kor J Mycol. 1977;5:27–31. [Google Scholar]
- 6.Chung KT, Seo SK, Song HI. Changes of polyphenols and polyphenol oxidase active bands during apple wine fermentation. Korean J Food Sci Technol. 1984;16:413–417. [Google Scholar]
- 7.Chung JH, Mok C, Lim S, Park YS. Ultrafiltration for quality improvement of apple wine. J Korean Soc Agric Chem Biotechnol. 2003;46:201–206. [Google Scholar]
- 8.Choi SH, Baek SY, Yeo SH, Park HD. Rapid fermentation of freeze-concentrated ice apple wine by a sugar tolerant yeast, Saccharomyces cerevisiae SS89. Korean J Food Preserv. 2012;19:413–419. doi: 10.11002/kjfp.2012.19.3.413. [DOI] [Google Scholar]
- 9.Lee JH, Kang TH, Um BH, Sohn EH, Han WC, Ji SH, Jang KH. Evaluation of physicochemical properties and fermenting qualities of apple wines added with medicinal herbs. Food Sci Biotechnol. 2013;22:1039–1046. doi: 10.1007/s10068-013-0181-y. [DOI] [Google Scholar]
- 10.Patel S, Shibamoto T. Effect of 20 different yeast strains on the production of volatile components in Symphony wine. J Food Comp Anal. 2003;16:469–476. doi: 10.1016/S0889-1575(03)00021-8. [DOI] [Google Scholar]
- 11.Jang JH, Yi SH, Kim JH, Lee DH, Lee JS. Effects of Vitis coignetiae on the quality and antihypertension of Vitis hybrid red wine. Korean J Microbiol Biotechnol. 2011;39:126–132. [Google Scholar]
- 12.Masneuf-Pomarède I, Mansour C, Murat ML, Tominaga T, Dubourdieu D. Influence of fermentation temperature on volatile thiols concentrations in Sauvignon blanc wines. Int J Food Microbiol. 2006;108:385–390. doi: 10.1016/j.ijfoodmicro.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Danilewicz JC, Seccombe JT, Whelan J. Mechanism of interaction of polyphenols, oxygen, and sulfur dioxide in model wine and wine. Am J Enol Vitic. 2008;59:128–136. [Google Scholar]
- 14.Lester MR. Sulfite sensitivity: significance in human health. J Am Coll Nutr. 1995;14:229–232. doi: 10.1080/07315724.1995.10718500. [DOI] [PubMed] [Google Scholar]
- 15.Vally H, Misso NL, Madan V. Clinical effects of sulphite additives. Clin Exp Allergy. 2009;39:1643–1651. doi: 10.1111/j.1365-2222.2009.03362.x. [DOI] [PubMed] [Google Scholar]
- 16.Versari A, Parpinello GP, Cattaneo M. Leuconostoc oenos and malolactic fermentation in wine: a review. J Ind Microbiol Biot. 1999;23:447–455. doi: 10.1038/sj.jim.2900733. [DOI] [Google Scholar]
- 17.Peng B, Yue T, Yuan Y. Analysis of key aroma components in cider from Shaanxi (China) Fuji apple. Int J Food Sci Technol. 2009;44:610–615. doi: 10.1111/j.1365-2621.2008.01875.x. [DOI] [Google Scholar]
- 18.Wang D, Xu Y, Hu J, Zhao G. Fermentation kinetics of different sugars by apple wine yeast Saccharomyces cerevisiae. J Inst Brew. 2004;110:340–346. doi: 10.1002/j.2050-0416.2004.tb00630.x. [DOI] [Google Scholar]
- 19.Cheirsilp B, Umsakul K. Processing of banana-based wine product using pectinase and α-amylase. J Food Process Eng. 2008;31:78–90. doi: 10.1111/j.1745-4530.2007.00152.x. [DOI] [Google Scholar]
- 20.Jayani RS, Saxena S, Gupta R. Microbial pectinolytic enzymes: a review. Process Biochem. 2005;40:2931–2944. doi: 10.1016/j.procbio.2005.03.026. [DOI] [Google Scholar]
- 21.Yi SH, Ann YG, Choi JS, Lee JS. Development of peach fermented wine. Korean J Food & Nutr. 1996;9:409–412. [Google Scholar]
- 22.Jarvis B. Cider, perry, fruit wines and other alcoholic fruit beverages. In: Arthey D, Ashust PR, editors. Fruit Processing. Springer US; New York, NY, USA: 1996. pp. 97–134. [DOI] [Google Scholar]
- 23.Bae SM. Home brewing using fruits and herbs. Baesanmyun Brewing Research Center; Seoul, Korea: 2002. pp. 17–44. [Google Scholar]
- 24.NTS. Regulations of liquor analysis. National Tax Service; Sejong, Korea: 2009. pp. 12–41. [Google Scholar]
- 25.Kim HR, Lee AR, Kwon YH, Lee HJ, Jo SJ, Kim JH, Ahn BH. Physicochemical characteristics and volatile compounds of glutinous rice wines depending on the milling degrees. Korean J Food Sci Technol. 2010;42:75–81. [Google Scholar]
- 26.Lee DH, Jung JW, Lee YS, Seo JS, Park IT. Fermentation characteristics for preparation of distilled liquor made of mixed grains. Korean J Food Sci Technol. 2014;46:446–455. doi: 10.9721/KJFST.2014.46.4.446. [DOI] [Google Scholar]
- 27.Park JH, Bae SM, Yook C, Kim JS. Fermentation characteristics of Takju prepared with old rice. Korean J Food Sci Technol. 2004;36:609–615. [Google Scholar]
- 28.Park CS, Lee TS. Quality characteristics of takju prepared by wheat flour nuruks. Korean J Food Sci Technol. 2002;34:296–302. [Google Scholar]
- 29.So MH, Lee YS, Noh WS. Improvement in the quality of Takju by a modified Nuruk. Korean J Food & Nutr. 1999;12:427–432. [Google Scholar]
- 30.Jin TY, Chung HJ, Eun JB. The effect of fermentation temperature on the quality of Jinyangju, a Korean traditional rice wine. Korean J Food Sci Technol. 2006;38:414–418. [Google Scholar]
- 31.Yook C, Seo MH, Lee JW, Kim YH, Lee KY. Quality properties of wines fermented with domestic new different grapes. Korean J Food Sci Technol. 2008;40:633–642. [Google Scholar]
- 32.Satora P, Tarko T, Duda-Chodak A, Sroka P, Tuszyński T, Czepielik M. Influence of prefermentative treatments and fermentation on the antioxidant and volatile profiles of apple wines. J Agric Food Chem. 2009;57:11209–11217. doi: 10.1021/jf9025053. [DOI] [PubMed] [Google Scholar]
- 33.Youn KS, Hong JH, Bae DH, Kim SJ, Kim SD. Effective clarifying process of reconstituted apple juice using membrane filtration with filter-aid pretreatment. J Membr Sci. 2004;228:179–186. doi: 10.1016/j.memsci.2003.10.006. [DOI] [Google Scholar]
- 34.Whiting GC. Organic acid metabolism of yeasts during fermentation of alcoholic beverages–a review. J Inst Brew. 1976;82:84–92. doi: 10.1002/j.2050-0416.1976.tb03731.x. [DOI] [Google Scholar]
- 35.Kim E, Chang YH, Ko JY, Jeong Y. Quality characteristics of Makgeolli added with kiwifruit (Actinidia deliciosa) J Korean Soc Food Sci Nutr. 2013;42:1821–1828. doi: 10.3746/jkfn.2013.42.11.1821. [DOI] [Google Scholar]
- 36.Lilly M, Lambrechts MG, Pretorius IS. Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl Environ Microbiol. 2000;66:744–753. doi: 10.1128/AEM.66.2.744-753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doco T, Williams P, Cheynier V. Effect of flash release and pectinolytic enzyme treatments on wine polysaccharide composition. J Agric Food Chem. 2007;55:6643–6649. doi: 10.1021/jf071427t. [DOI] [PubMed] [Google Scholar]
- 38.Viegas CA, Sá-Correia I. Effects of low temperatures (9~33°C) and pH (3.3~5.7) in the loss of Saccharomyces cerevisiae viability by combining lethal concentrations of ethanol with octanoic and decanoic acids. Int J Food Microbiol. 1997;34:267–277. doi: 10.1016/S0168-1605(96)01200-7. [DOI] [PubMed] [Google Scholar]
- 39.Kim JS, Kim SH, Han JS, Yoon BT, Yook C. Effects of sugar and yeast addition on red wine fermentation using Campbell Early. Korean J Food Sci Technol. 1999;31:516–521. [Google Scholar]
- 40.Carreón-Valencia T. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 71 Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. International Agency for Research on Cancer; Lyon, France: 1999. Acetaldehyde; pp. 319–328. [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy LVA, Reddy OVS. Production and characterization of wine from mango fruit (Mangifera indica L) World J Microbiol Biotechnol. 2005;21:1345–1350. doi: 10.1007/s11274-005-4416-9. [DOI] [Google Scholar]