Extended Data Figure 6. In vivo P. berghei mouse-to-mouse assay.
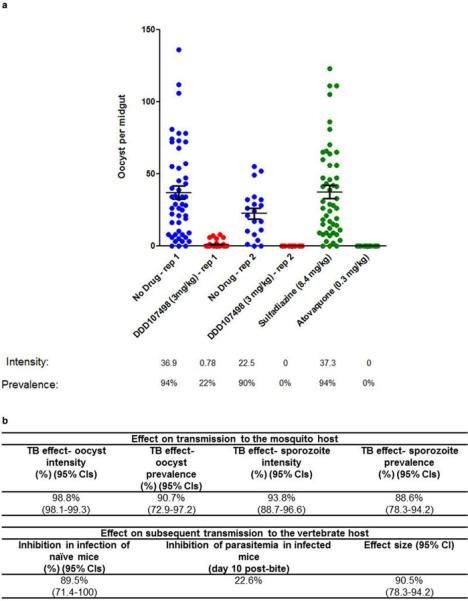
a. Impact of DDD107498 treatment on in vivo mosquito infection. Mice infected with P. berghei (PbGFPCON507 clone 1)19 were dosed orally with either DDD107489 at 3 mg/kg, atovaquone at 0.3 mg/kg, sulphadiazine at 8.4 mg/kg, or were not drug treated (negative control). After 24 h, populations of treated mice (n=5) were exposed to 500 female A. stephensi mosquitoes, and oocyst intensity and infection prevalence in the mosquito midgut was measured 10 days post-feeding. Individual data points represent the number of oocysts found in individual mosquitoes. Two replicates were performed. Sulphadiazine (8.4 mg/kg, i.p.) and atovaquone (0.3 mg/kg, i.p.) were used as negative and positive transmission-blocking drug controls, respectively. Horizontal bars indicate mean intensity of infection, whilst error bars indicate S.E.M. within individual mosquito populations. b. Impact of treatment with DDD107498 over a complete transmission cycle in vivo. Following drug treatment of infected mice and mosquito feeds, surviving potentially infectious mosquitoes were allowed to blood-feed on naive mice at a range of transmission settings (biting rates of 2,5 and 10 bites per naive mouse) to assess ability of drug treatment to reduce the number of new malarial cases post mosquito bite. Efficacy is expressed as: i) impact on the mosquito population – expressed as reduction in both oocyst and sporozoite intensity and prevalence; ii) impact on subsequent transmission to new naïve vertebrate hosts – expressed as reduction in infection of naïve mice (reduction in number of new malarial cases post-mosquito bite) and inhibition of subsequent parasitema (day 10 post-bite) in mice that do become infected; iii) Effect size generated – by fitting the data achieved within this assay to a chain-binomial model we can assess the ability of DDD107498 to reduce Ro (assuming 100% coverage). If Ro is reduced to <1, transmission is unsustainable and elimination will occur. 95% Cls are shown in brackets. Efficacy is calculated in comparison with no-drug controls. TB is transmission blocking
