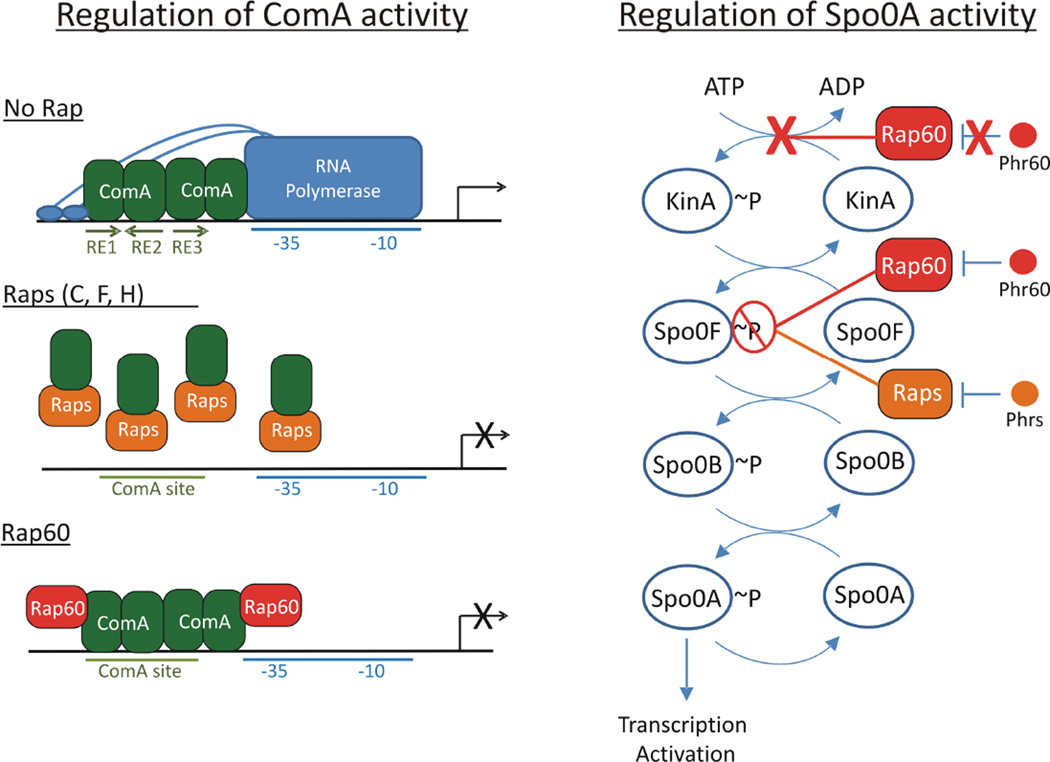
Fig. 9.
Model of ComA and Spo0A regulation by Rap proteins.
A. Two proposed mechanisms of ComA anti-activation by Rap proteins. In the absence of Rap proteins, ComA binds to a tripartite binding sequence located in the promoter region of target genes and recruits RNA polymerase to activate transcription of target genes. Raps C, F and H bind to the DNA binding domain of ComA, inducing a comformational change that disrupts ComA dimerization and interferes with ComA binding to DNA. In contrast, Rap60 forms a ternary complex with ComA and DNA that inhibits the activity of ComA without perturbing binding to DNA. We speculate that Rap60 binds to a region of ComA that prevents ComA access to the transcriptional machinery. Phr peptides antagonize their cognate Rap proteins allowing ComA-dependent transcription activation of target genes.
B. Rap proteins regulate Spo0A by modulating the sporulation phosphorelay system. Rap proteins, including Rap60, act as phosphatases of Spo0F~P. Dephosphorylation of Spo0F~P prevents the phosphorylation of Spo0A and reverses the flow of phosphate through the phosphorelay system resulting in decreased levels of Spo0B~P and Spo0A~P. Phr peptide antagonizes Rap phosphatase activity of Spo0F~P resulting in the accumulation of Spo0A~P and transcription activation of early sporulation genes. Rap60 has an additional, noncanonical role in regulating Spo0A activity by inhibiting the autophosphorylation of KinA. Phr60 has no effect on this activity by Rap60.