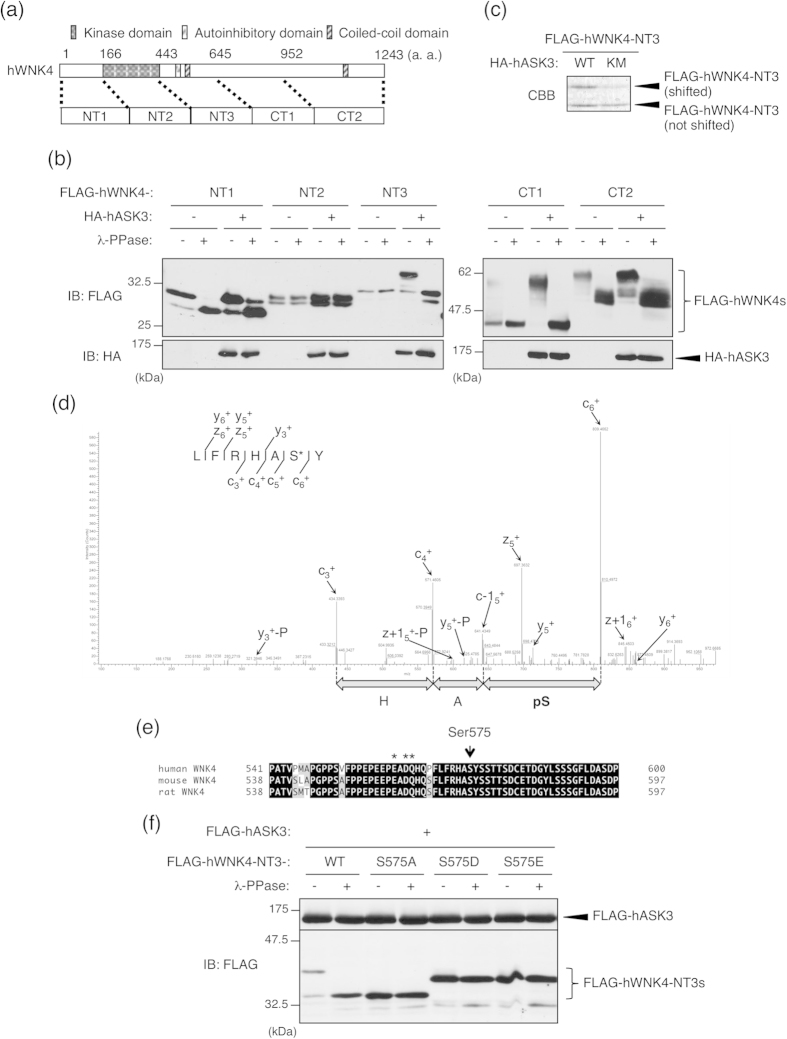
Figure 2. WNK4 Ser575 is an ASK3-dependent phosphorylation site.
(a) A schematic view of WNK4. We constructed five FLAG-tagged WNK4 fragments (NT1/2/3, CT1/2) as indicated. (b) Mobility shift assay to narrow down the region containing ASK3-dependent phosphorylation sites. HEK293A cells were transiently transfected with HA-hASK3 and FLAG-tagged WNK4 fragments. Lysates were divided, and a portion was treated with λ-PPase for 30 min at 30 °C. The reaction was terminated by the addition of SDS sample buffer, and samples were subjected to IB. As a result, bands corresponding to fragments NT3 and CT1 were shifted higher following co-expression of ASK3, and this shift was abolished by λ-PPase treatment. Full-length blots are presented in Supplementary Fig. S6a. (c) HEK293A cells were transiently transfected with FLAG-hWNK4-NT3 and HA-hASK3-WT/KM. Cells were lysed at 48 h after transfection, and FLAG-hWNK4-NT3 proteins were immunoprecipitated with anti-FLAG antibody beads. Samples were subjected to SDS-PAGE and stained with CBB. (d) The ETD-MS/MS spectrum of peptide 138–143 from a chymotryptic digest of FLAG-hWNK4-NT3, localizing phosphorylation to Ser142 in the construct, which corresponds to Ser575 of human WNK4. Asterisk indicates identified phosphorylated residues. (e) Alignment of WNK4 orthologues. The Ser575 residue in human WNK4 (arrow) is conserved in murine WNK4 orthologues and is the nearest serine residue to the PHAII mutation sites (asterisks). (f) Ser575 phosphorylation is essential for the ASK3-dependent mobility shift of the NT3 fragment. HEK293A cells were transfected with FLAG-hASK3 and each FLAG-tagged hWNK4-NT3 mutants (WT, S575A, S575D and S575E). Lysates were divided and a portion was treated with λ-PPase for 30 min at 30 °C. The reaction was terminated by the addition of SDS sample buffer, and samples were subjected to IB. Full-length blots are presented in Supplementary Fig. S6b.