Figure 6. Ser575 phosphorylation is not critically involved in the regulation of WNK4 kinase activity and the binding affinity to KLHL3.
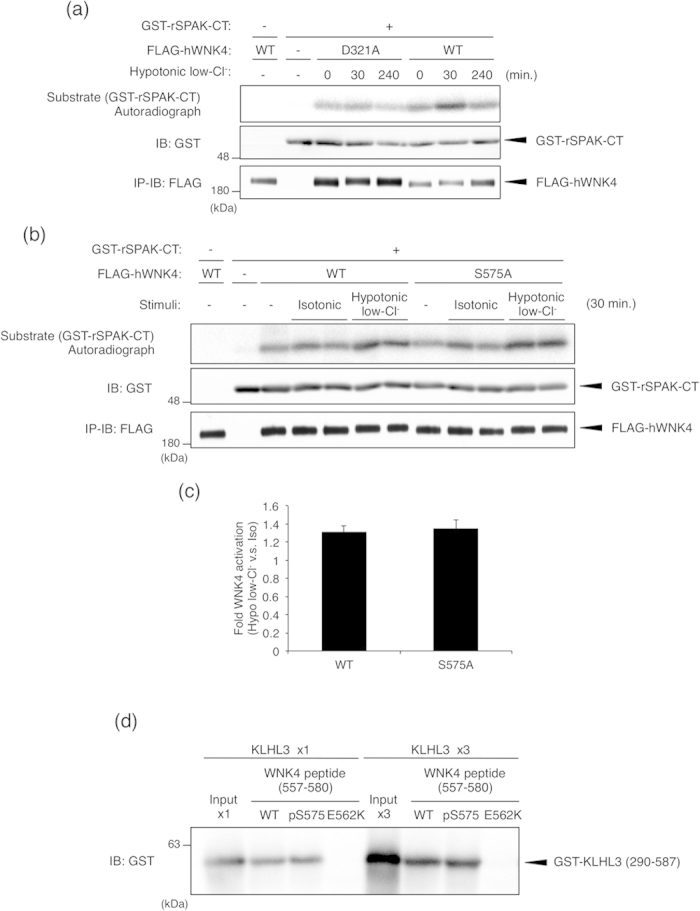
(a) WNK4 kinase activity is increased in a hypotonic low-chloride stimulation-dependent manner. mpkDCT cells were infected with adenoviral vectors encoding FLAG-hWNK4-WT or the D321A mutant. At 48 h after infection, cells were lysed, and FLAG-tagged WNK4 proteins were immunoprecipitated with anti-FLAG antibody beads. Immunoprecipitated kinases were incubated with GST-rSPAK-CT (348–553) and Mg-ATP for 15 min at 30 °C. The reaction was terminated by the addition of SDS sample buffer, and samples were subjected to autoradiogram and IB. Full-length blots are presented in Supplementary Fig. S12a. (b) WNK4-WT and the WNK4-S575A mutant are equally activated by hypotonic low-chloride stimulation. mpkDCT cells were infected with adenoviral vectors encoding FLAG-hWNK4-WT or the S575A mutant. At 48 h after infection, cells were treated with isotonic or hypotonic low-Cl− stimulation for 30 min. Then, cells were lysed, and FLAG-tagged WNK4 proteins were immunoprecipitated with anti-FLAG antibody beads. Immunoprecipitated kinases were incubated with GST-rSPAK-CT (348–553) and Mg-ATP for 15 min at 30 °C. The reaction was terminated by the addition of SDS sample buffer, and samples were subjected to autoradiogram and IB. Full-length blots are presented in Supplementary Fig. S12b. (c) Quantification of the hypotonic low-chloride stimulation-dependent WNK4 activation. The fold activation was calculated by dividing the intensity of autoradiogram in hypotonic low-Cl− stimulation by that in isotonic stimulation in Fig. 5b. Error bars indicate SEM (N = 5). (d) In vitro interaction between KLHL3 and WNK4 (557–580) peptides. GST-KLHL3 (290–587) protein was pulled-down with beads that were coated with synthesized WNK4 (557–580) peptides. WT: the unphosphorylated peptide, pS575: the phosphorylated peptide at Ser575 and E562K: the peptide harboring PHAII mutation (a Glu to Lys substitution at 562 a.a.) as a negative control. The amount of pulled-down GST-KLHL3 was analyzed by IB. The input lane shows the total amount of GST-KLHL3 applied to the pull-down system. In the right four lanes, 3-fold higher amount of GST-KLHL3 was applied compared to that in the left four lanes. Full-length blots are presented in Supplementary Fig. S12c.
