Figure 8. PDE1C is associated with PDGFRβ.
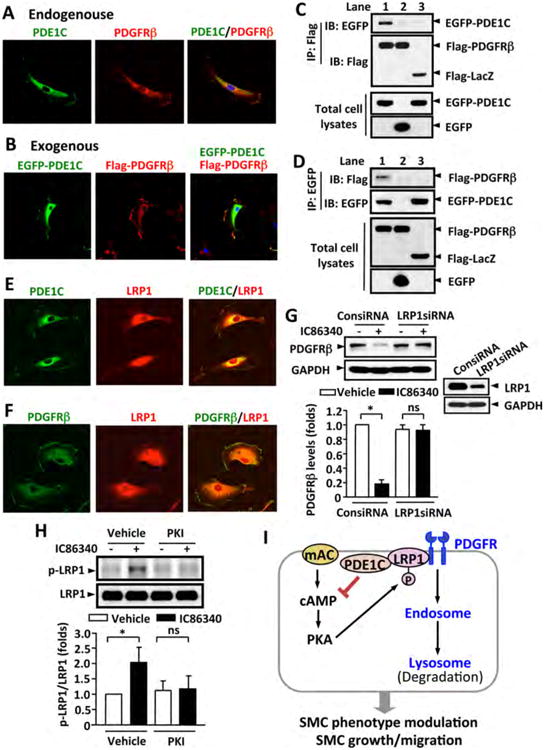
(A) Representative images showing endogenous PDE1C and PDGFRβ co-localized on cytoplasmic membrane. Rat aortic SMCs were immunostained with anti-PDE1C and anti-PDGFRβ antibodies. (B) Representative images showing exogenous PDE1C and PDGFRβ co-localization on plasma membrane. Rat aortic SMCs were co-transfected with Flag-PDGFRβ and EGFP-PDE1C by electroporation, and exogenous Flag-PDGFRβ and EGFP-PDE1C were detected by immunostaining with anti-flag and anti-GFP antibodies, respectively. Cells were visualized using confocal microscopy. (C-D) Co-immunoprecipitation (IP) revealed that PDE1C and PDGFRβ associate together. HEK293A cells were co-transfected with Flag-PDGFRβ and EGFP-PDE1C (lane 1), Flag-PDGFRβ and EGFP (lane 2), or Flag-LacZ and EGFP-PDE1C (lane 3), C, IP with anti-Flag antibody and IB with anti-EGFP or anti-Flag antibody. D, IP with anti-EGFP antibody and IB with anti-Flag or anti-EGFP antibody. The expression of Flag-tagged and EGFP proteins in total cell lysates were immunoblotted with anti-Flag or anti-EGFP antibody, respectively. (E-F) Representative images showing the co-localization of PDE1C and LRP1 (E) or PDGFRβ and LRP1 (F) by immunofluorescent staining. Rat aortic SMCs were immunostained with anti-PDE1C or anti-PDGFRβ together with anti-LRP1 antibodies. (G) Knockdown of LRP-1 attenuated IC86340-induced PDGFRβ degradation. Rat aortic SMCs were transfected with 50 nmol/L control siRNA (ConsiRNA) or LRP1 siRNA (LRP1siRNA) for 2 days, followed by treatment with 15 μmol/L IC86340 in DMEM supplied with 0.1% FBS for 24 h. The protein levels were determined by western blot. (H) PDE1 inhibitor IC86340 stimulates LRP1 phosphorylation in a PKA-dependent manner. Rat aortic SMCs were treated with vehicle or 15 μmol/L IC86340 for 12 h in the presence of vehicle or 5 μ mol/L PKI (14-22) peptide. The phosphorylation of LRP1 was detected by immunoblotting with a phospho-PKA substrate antibody after immunoprecipitation with LRP1 antibody. Values are mean ± SD of (n=3-4). *P < 0.05; ns: no significant difference. (I) Proposed model: a tmAC-derived cAMP-PKA signaling is critical in promoting PDGFRβ internalization and endocytosis in a LRP1-dependent manner.
