Abstract
Because of the toxicity associated with myeloablative conditioning, nonmyeloablative regimens are increasingly being used in vulnerable patient populations. For patients with sickle cell disease, stable mixed chimerism has proven sufficient to reverse the phenotype. Because the vast majority of patients do not have an HLA-matched sibling, a safe nonmyeloablative regimen that could be applied to the haploidentical setting would be ideal. We employed a mismatched mouse model using BALB/c donors and C57BL/6 recipients. Recipient mice were conditioned with 200 cGy TBI and sirolimus or CSA with or without post transplant Cy (PT-Cy). Our data show that when sirolimus or PT-Cy alone is given to C57BL/6 recipients, donor cells are not detected. However, when sirolimus is administered for 15 or 31 days starting 1 day before or up to 6 days after transplant with PT-Cy, all mice maintain stable mixed chimerism. In contrast, conventional therapy employing CSA with or without PT-Cy does not result in stable mixed chimerism. Lastly, mice with stable mixed chimerism after sirolimus display decreased reactivity to donor Ag both in vitro and in vivo. These data identify a novel strategy for inducing mixed chimerism for the treatment of nonmalignant hematologic diseases.
Keywords: sirolimus, Cy, CSA, mixed chimerism, murine
INTRODUCTION
Hematopoietic SCT is the only curative option for patients with severe congenital anemias including β-thalassemia and sickle cell disease (SCD). Myeloablative conditioning has been successful in pediatric patients.1–3 However, myeloablative protocols have been avoided in many adult patients with SCD because of high risk of transplant-related mortality. Stable mixed chimerism as low as 11% in patients with SCD was sufficient to reverse the phenotype.4 We developed a nonmyeloablative regimen that has been successful for patients who have HLA matched sibling donors.5 Despite our awareness of matched sibling availability for some patients before enrollment, we identified suitable matched-sibling donors in only 21% of 112 recruited patients. Matched unrelated donor and umbilical cord blood donor sources are similarly limited.6 We therefore sought to develop a nonmyeloablative haploidentical PBSC transplant regimen.
In vitro data have shown that the mammalian target of rapamycin (mTOR) inhibitor sirolimus, unlike CSA, leads to T-cell anergy and promotes the generation of regulatory T cells (T-regs).7–10 We confirmed this in an in vivo F1-into-parent model favoring graft rejection where recipient mice were conditioned with 300 cGy TBI and received 100 × 106 donor splenocytes.11 Mice that received CSA rejected their grafts during the 4-week period of immunosuppression. However, mice that received sirolimus maintained their grafts for at least 7 months after discontinuing immunosuppressive therapy. Similarly, in a rat BMT model, rats that received 600 or 800 cGy TBI with CSA only transiently engrafted,12 while rats that received both doses of TBI with sirolimus achieved stable mixed chimerism. More intense regimens of lymphocyte depletion plus sirolimus have permitted even lower TBI doses in mice to achieve high chimerism while preserving functional tolerance.13 Sirolimus-based conditioning has also induced mixed chimerism in a rhesus macaque SCT model.14
While we have shown that sirolimus in addition to TBI and alemtuzumab allows stable mixed chimerism in the HLA-matched sibling setting,5 additional immunosuppressive therapy will likely be required in the haploidentical setting because of an increased risk for GVHD. Emerging data suggest that post transplant Cy (PT-Cy) is an effective strategy that can successfully be used in patients with malignant and nonmalignant hematologic diseases in the haploidentical setting.15–17 Cy given 2–3 days post transplant is thought to promote tolerance induction by deleting alloreactive lymphocytes.18–23 We sought to determine whether PT-Cy would improve engraftment when administered with sirolimus and/or CSA by employing a stringent MHC-mismatched mouse model using BALB/c donors and C57BL/6 recipients.
MATERIALS AND METHODS
Mice and transplants
Female BALB/c (H2Kd) donor, male C57BL/6 (H2Kb) recipient and female nonobese diabetic (NOD) (H2Abg7) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) or Taconic (Hudson, NY, USA). Mice ranged in age from 6 to 10 weeks at the time of transplant. All mice were handled and cared for according to the Animal Care and Use Protocol, which was approved by the Animal Care and Use Committee at the National Institutes of Health. Where noted, TBI was given on the day of transplant, and immunosuppressive therapy with sirolimus or CSA was given for 15 or 31 days. PT-Cy was administered as a single dose 2 days post transplant. Femurs and tibias from BALB/c donor mice were flushed, RBCs were lysed using ACK (ammonium–chloride–potassium) lysing buffer (Quality Biological, Gaithersburg, MD, USA) and 4–5 recipient mice per group received 22–25 × 106 BM cells intravenously into the tail vein with 300 μL of vehicle. Following transplant, mice were monitored for survival and signs of morbidity (weight loss, ruffled fur, decreased energy and hunched posture). Donor chimerism levels were checked via flow cytometry every 1 to 2 months until 5 to 12 months post transplant. PB from recipient mice was stained with an FITC-conjugated MoAb to the Kd Ag and a phycoerythrin-conjugated MoAb to the Kb Ag (BD Biosciences, San Jose, CA, USA) and analyzed on a BD-FacsCalibur (BD Biosciences) flow cytometer (Supplementary Figure 2).
Immunosuppressive agents
Sirolimus (Wyeth Pharmaceuticals, Philadelphia, PA, USA) 2 mg tablets were crushed and suspended in carboxymethyl cellulose (Sigma-Aldrich, St Louis, MO, USA) and polysorbate 80 (Sigma-Aldrich). Sirolimus was administered at a dose of 3 mg/kg per day intraperitoneally. Lyophilized Cy (Baxter, Deerfield, IL, USA) was reconstituted with normal saline to make a stock solution of 20 mg/mL. Cy was given at a dose of 200 mg/kg per day intraperitoneally. CSA (Calbiochem, San Diego, CA, USA) was solubilized in carboxymethyl cellulose and polysorbate 80, a stock solution of 10 mg/mL was made and mice were given a dose of 5–20 mg/kg per day intraperitoneally.
MLR
Spleens were harvested from mice, mashed, filtered and drawn repeatedly through a 25 G needle to ensure single-cell suspension. Responder CD4 + cells were isolated via MACS separation (Miltenyi Biotec, Auburn, CA, USA) as per the manufacturer’s recommendations following overnight culture of the splenocytes in RPMI +10% FBS. Stimulator splenocytes were irradiated at 25 Gy. MLR was run in triplicate with 1 × 105 responders and 1 × 105 stimulators per 200 μL well (1:1 ratio) and incubated. On day 4, 25 μL of media containing 1μCi of 3H-thymidine was added to each well with agitation. Cells were harvested onto a FilterMate filter paper on day 5 and c.p.m. were read on a MicroBeta 96-well scintillation counter (Perkin-Elmer, Waltham, MA, USA).
Skin graft
Skin graft procedures were carried out on a sterile stage using sterile instruments. Mice were provided with buprinex two times daily beginning the morning before skin graft and continuing for 1 week. On the afternoon of the procedure, ears were removed from BALB/c mice immediately after euthanasia and split to obtain the dorsal skin tissue. Tissues were held in a dish of ice-cold sterile saline until ready to transplant. Skin graft recipient mice were anesthetized by isoflourane inhalation per standard procedures, oriented laterally and the operative site cleared with depilatory cream. The site was washed three times, alternating each scrub with povidone iodine and 70% isopropyl alcohol as per standard procedures. An incision was created in the thorax of the recipient mouse to create a 1-cm diameter wound approximating the size of the donor tissue. Donor tissue was placed over the wound and held in place with three single 7-0 Prolene (Ethicon, Cornelia, GA, USA) sutures. A standard sterile adhesive bandage was wrapped around the mouse and held closed with a suture. After recovery from anesthesia, mice were individually housed in sterile cages and monitored two times daily. Bandages were replaced as needed until 8 days after the procedure.
RESULTS
Sirolimus and PT-Cy are synergistic in an MHC-mismatched mouse model
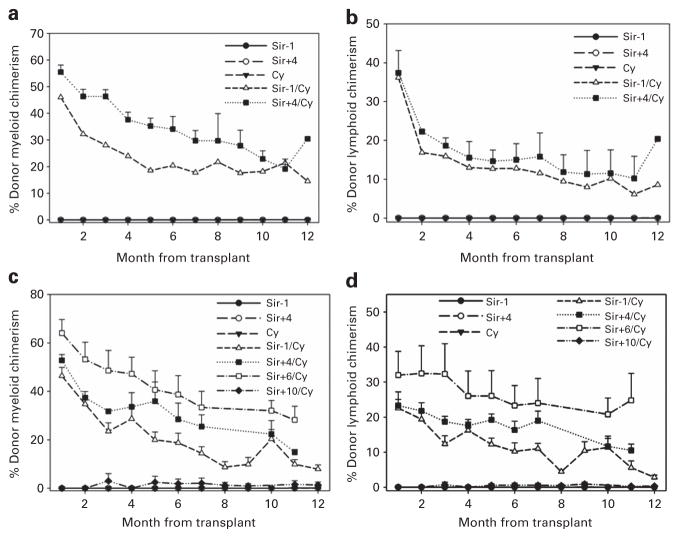
Previous studies demonstrated that the combination of 200 cGy TBI, 200 mg/kg per day PT-Cy and 25 × 106 BM cells was insufficient to induce mixed chimerism in a mismatched mouse model.19,20 Thus, we investigated whether the addition of sirolimus would induce stable mixed chimerism. Experiments were also designed to introduce sirolimus at various time points to determine its potential for toxicity and/or synergistic combination with PT-Cy. In all, 20 recipient mice received sirolimus 3 mg/kg per day intraperitoneally for 31 days starting 1 day before or 4 days after transplant with or without PT-Cy 200 mg/kg intraperitoneally given 2 days after transplant. On the day of transplant, mice received 200 cGy TBI followed by 25 × 106 BM cells. All mice that received sirolimus and PT-Cy engrafted, regardless of whether sirolimus was given before or after transplant (Figures 1a and b). Donor myeloid chimerism levels initially reached as high as 55% during the period of immunosuppression, and later stabilized at 15–30% in engrafted mice (Figure 1a). Corresponding lymphoid chimerism levels were about 10–20% (Figure 1b) even 11 months after sirolimus was discontinued. All mice that received only sirolimus or PT-Cy failed to stably engraft.
Figure 1.
Donor chimerism levels in mice that receive sirolimus (Sir) with or without post transplant Cy. C57BL/6 mice (n =20) received 200 cGy TBI, 25 × 106 BM cells and Sir (3 mg/kg per day intraperitoneally (i.p.)) for 31 days starting 1 day before or 4 days after transplant with or without Cy (200 mg/kg i.p.) given 2 days post transplant. (a) Donor myeloid chimerism levels. (b) Donor lymphoid chimerism levels. C57BL/6 mice (n =38) received 200 cGy TBI, 22–25 × 106 BM cells and Sir (3 mg/kg per day i.p.) for 15 days starting 1 day before or 4, 6 or 10 days after transplant with or without Cy (200 mg/kg i.p.) given 2 days post transplant. (c) Donor myeloid chimerism levels. (d) Donor lymphoid chimerism levels. These data represent a compilation of two experiments.
Fifteen days of sirolimus and PT-Cy are sufficient to maintain mixed chimerism
We next sought to determine whether a shorter interval of immunosuppressive therapy would be sufficient to support stable mixed chimerism and whether sirolimus could be introduced at later time points after transplant. Recipient mice received 3 mg/kg per day sirolimus from 1 day before to 10 days after transplant with or without PT-Cy 200 mg/kg 2 days post transplant. On the day of transplant, recipient mice received 200 cGy TBI and 22–25 × 106 BM cells. All mice that received sirolimus from 1 day before to 6 days after transplant and PT-Cy maintained mixed chimerism (Figures 1c and d). Donor myeloid chimerism levels ranged from 45 to 65% at 1 month post transplant, and then between 10 and 30% up to 12 months post transplant (Figure 1c). Only 1 of 5 mice that received sirolimus for 15 days starting 10 days after transplant and PT-Cy engrafted with donor chimerism levels ranging between 5 and 30%. Engrafted mice also displayed lower but stable mixed lymphoid chimerism (Figure 1d). Again, donor chimerism was not detected in mice that received only sirolimus or PT-Cy.
CSA prevents mixed chimerism induction
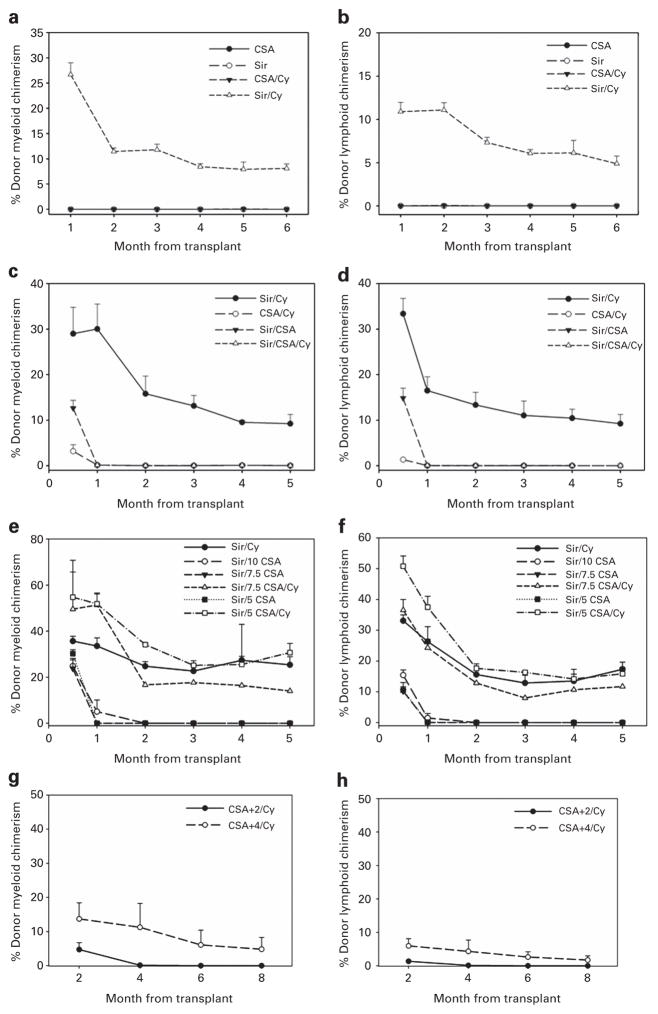
We have shown previously that CSA inhibits stable mixed chimerism in an F1 into parent model.11 We next evaluated whether CSA and PT-Cy would allow stable mixed chimerism in our mismatched model. Recipient mice received sirolimus 3 mg/kg per day or CSA 20 mg/kg per day for 15 days starting 1 day before transplant with or without PT-Cy 200 mg/kg 2 days after transplant. On the day of transplant, mice received 200 cGy TBI and 25 × 106 BM cells. Only mice receiving sirolimus and PT-Cy displayed myeloid (Figure 2a) and lymphoid (Figure 2b) donor engraftment. All mice that received CSA, alone or in combination with PT-Cy, failed to engraft. As expected, only mice that received sirolimus and PT-Cy maintained donor chimerism levels consistent with previous data.
Figure 2.
Assessment of donor chimerism in mice that receive CSA and/or Sir with or without Cy. C57BL/6 mice (n =20) received 200 cGy TBI, 25 × 106 BM cells and either CSA (20 mg/kg per day intraperitoneally (i.p.)) or Sir (3 mg/kg per day i.p.) for 15 days with or without Cy (200 mg/kg i.p.) given 2 days post transplant. (a) Donor myeloid chimerism levels. (b) Donor lymphoid chimerism levels. C57BL/6 mice (n =24) received 200 cGy TBI, 25 × 106 BM cells, and Sir (3 mg/kg per day i.p.), CSA (10–20 mg/kg per day i.p.) and/or Cy (200 mg/kg i.p.) given 2 days post transplant. (c) Donor myeloid chimerism levels. (d) Donor lymphoid chimerism levels. C57BL/6 mice (n =30) received 200 cGy TBI, 25 × 106 BM cells, and Sir (3 mg/kg per day i.p.), CSA 5–10 mg/kg per day i.p.) and/or Cy (200 mg/kg i.p.) given 2 days post transplant. (e) Donor myeloid chimerism levels. (f) Donor lymphoid chimerism levels. C57BL/6 mice (n =10) received 200 cGy TBI, 25 × 106 BM cells and Cy given 2 days post transplant. 20 mg/kg per day CSA was administered for 15 days beginning 2 or 4 days post transplant. (g) Donor myeloid chimerism levels. (h) Donor lymphoid chimerism levels.
CSA given with sirolimus and PT-Cy is too toxic to evaluate donor chimerism levels
We next wanted to assess whether CSA would prevent sirolimus and PT-Cy-induced chimerism. Because sirolimus can increase the toxic effects of CSA,24–26 the CSA dose was decreased to 10 mg/kg per day while maintaining the sirolimus dose at 3 mg/kg per day, and the drugs were given intraperitoneally as two separate injections at least 4 h apart in mice receiving both. Twenty-four recipient mice received either sirolimus with PT-Cy, CSA 20 mg/kg per day with PT-Cy, sirolimus and CSA alone or both drugs with PT-Cy. All mice that received sirolimus and PT-Cy maintained mixed chimerism (Figures 2c and d). Mice that received CSA and sirolimus alone initially achieved myeloid and lymphoid chimerism levels of 13% and 15%, respectively, 2 weeks post transplant, but then lost their grafts by 4 weeks post transplant. Mice that received CSA with PT-Cy did not exhibit any donor chimerism. Mice that were treated with all three agents lost significant weight with hunching, lethargy and ruffled fur by 6 days post transplant. In response, dosing was reduced to every other day, which failed to prevent mortality, and therapy was discontinued by 10 days post transplant. Necropsy performed on one mouse revealed multibacterial pneumonia, attributed to severe myelosuppression. Analysis performed 4 weeks post transplant on the two remaining mice did not reveal donor cells.
This experiment was therefore repeated with reducing doses of CSA. A total of 30 mice were transplanted. Mixed chimerism was confirmed in mice that received sirolimus and PT-Cy (Figures 2e and f). Mice that received sirolimus and CSA with CSA doses ranging from 5 to 7.5 mg/kg per day displayed donor myeloid chimerism levels of 25–30% and lymphoid chimerism levels of 10% at 2 weeks post transplant. They subsequently rejected their grafts starting at 4 weeks post transplant. Mice that were administered sirolimus and a CSA dose of 10 mg/kg per day showed very low mixed chimerism at 4 weeks post transplant and lost their grafts by 8 weeks post transplant. Ten mice received all three drugs with CSA doses ranging from 5 to 7.5 mg/kg per day for 15 days starting 1 day before transplant. The mice again became ill around 5 days post transplant. One mouse from each group was bled 2 weeks post transplant, and donor myeloid chimerism levels ranged from 50 to 55% (Figure 2e) and lymphoid chimerism levels ranged from 35 to 50% (Figure 2f). The three surviving mice did maintain mixed donor myeloid and lymphoid chimerism at 5 months post transplant.
We additionally wished to determine whether the inhibition of T-cell proliferation elicited by CSA interferes with targeting of emerging alloreactive lymphocytes by PT-Cy. Mice were conditioned with 200 cGy TBI on the day of BMT and all mice received PT-Cy on day +2 as performed previously. CSA dosing was delayed until after transplant, but was given to all mice for 15 consecutive days. Of five mice that received CSA 4 days after transplant, one mouse showed donor chimerism at 18% myeloid (Figure 2g)/6.7% lymphoid (Figure 2h), which slowly dropped at 8 weeks post transplant, while the remaining mice failed to support their grafts (Figures 2g and h, combined data shown) as seen previously in mice receiving CSA immunosuppression. All mice that began receiving CSA 2 days after transplant failed to engraft.
Sirolimus-treated transplanted mice are functionally tolerant to donor
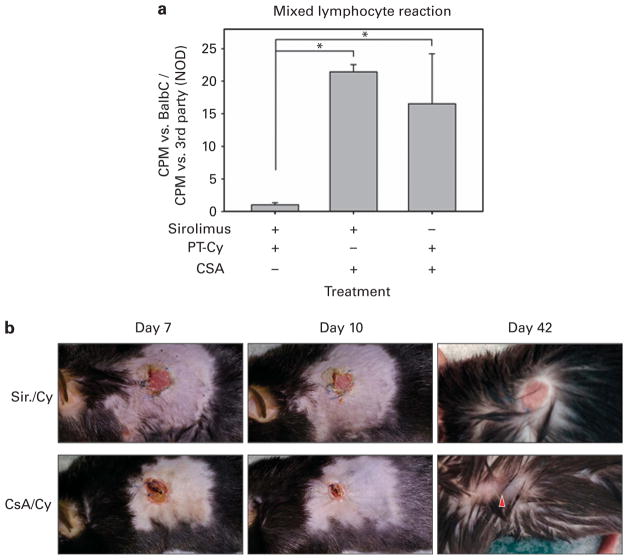
To investigate in vitro reactivity of the recovered lymphocytes to donor cells in our transplant models, spleens were removed from mice 5 to 6 months after transplant. Responder CD4 + T cells were isolated, and MLRs were performed against BALB/c (donor) and NOD (third party) irradiated splenocytes. Markedly decreased reactivity (P<0.05) to irradiated BALB/c stimulators was exhibited by CD4 + T cells from mice that had been treated intraperitoneally with sirolimus 3 mg/kg per day and PT-Cy as compared to mice receiving CSA 10 mg/kg per day with sirolimus or CSA 20 mg/kg per day with PT-Cy (Figure 3a).
Figure 3.
Sirolimus-treated transplanted mice are functionally tolerant to donor. Mixed lymphocyte reactions (a) were performed using responder CD4+ T cells isolated from spleens obtained from transplanted C57BL/6 mice treated with sirolimus +PT-Cy (n =3), sirolimus + CSA (n =2) and CSA +PT-Cy (n =2) and cultured with whole irradiated splenocytes obtained from BALB/c (donor) or NOD (third party) as described. Studies were performed 5 to 6 months post transplant. Data were analyzed as median c.p.m. (triplicate wells) of each responder vs BALB/c normalized to median c.p.m. vs NOD (positive control). Error bars represent s.e.m. One-way analysis of variance (ANOVA) with Student–Newman–Keuls multicomparison test was performed and asterisks denote statistical significance between sets (P<0.05). Skin graft surgery (b) was performed on C57BL/6 mice transplanted and treated as described with CSA +PT-Cy (n =3) or sirolimus (Sir.) +PT-Cy (n =4) 14 weeks post transplant using BALB/c ear tissue as the donor source. Representative images are shown. Days indicate day post skin graft. For 42-day images, fur was dampened to better reveal the graft site.
To confirm in vivo functional peripheral tolerance in our model, BALB/c skin grafts were placed 14 weeks post transplant on mice that had been treated with CSA +PT-Cy (n =3) or sirolimus +PT-Cy (n =4). The graft sites were photographed during healing (days 7–10 post-skin graft) and 6 weeks after the procedure (day 42). Acceptance of white, hairless BALB/c donor ear skin by sirolimus +PT-Cy mice was seen, while CSA +PT-Cy mice all exhibited gradual exclusion of donor tissue from the graft site, culminating with complete elimination weeks after the procedure and regrowth of black fur-bearing skin (Figure 3b).
DISCUSSION
Immunosuppression in transplantation commonly utilizes calcineurin inhibitors (tacrolimus and CSA) to inhibit TCR signaling. However, this may also prevent the induction of tolerance and generation of T-regs.27,28 Alternatively, sirolimus inhibits mTOR activation without blocking TCR-induced signaling and has been shown to decrease graft rejection and GVHD in murine models.29–31 Blocking the mTOR-signaling axis inhibits T cells from differentiating into Th1, Th2 or Th17 effector cells32 and promotes their differentiation into Foxp3 + T-regs.33,34 Supporting these observations, the ex vivo generation of such cells has been employed to mitigate graft rejection and GVHD in vivo,35,36 mice receiving sirolimus during SCT demonstrated tolerance induction37–39 and T-regs in the presence of sirolimus can permit chimerism even in the absence of standard cytoreductive conditioning in mismatched murine transplants.36 Functional tolerance was observed in our clinical experience, as 9 of the first 10 patients with severe SCD who underwent HLA-matched sibling PBSC transplant exhibit stable mixed WBC chimerism, donor-type Hb and phenotype reversal,5 and four have been weaned off of the sirolimus.
PT-Cy when given from 2 to 3 days post transplant is thought to decrease the incidence of graft rejection and GVHD by targeting alloreactive lymphocytes.18–23,40 Despite the antiproliferative effects of sirolimus, we have demonstrated that PT-Cy and sirolimus are synergistic at preventing graft rejection after lower-intensity TBI without lymphocyte depletion. However, profound lymphocyte depletion did not influence the efficacy of PT-Cy (Supplementary Figure 1). Interestingly, delaying sirolimus as long as 6 days post transplant effectively promoted stable mixed chimerism following PT-Cy. However, the majority of mice that were administered sirolimus starting 10 days post transplant did not develop mixed chimerism. Presumably, delaying sirolimus until after transplantation may permit enhanced donor cell expansion without the myelosuppressive effects of sirolimus. However, this must be balanced with the observation that the presence of sirolimus is important for permitting mixed chimerism post transplant. Despite improved donor chimerism when sirolimus is first given 4 and 6 days post transplant, we gave sirolimus starting 1 day before transplant in subsequent experiments to more closely model our clinical experience. Our results also demonstrate that 15 days of sirolimus therapy is sufficient to induce stable mixed chimerism in a murine model.
We have shown that in contrast to CSA, cells cultured and stimulated in the presence of sirolimus were rendered anergic.9 These results have also been confirmed in vivo where all mice or rats that received CSA rejected their grafts.11,12 In a recent clinical report employing a myeloablative regimen and a model prone to the development of GVHD, PT-Cy in the context of long-term CSA immunosuppression was found to decrease the incidence of GVHD.41 However, in our rejection model and using a nonmyeloablative regimen, only sirolimus induced long-term mixed chimerism despite the mice having received only sirolimus for a period of up to 30 days. Hale and co-workers37–39 have shown that sirolimus was superior to CSA at extending skin graft survival using fully mismatched and xenogeneic recipients, and sirolimus was also superior to CSA when administered with antilymphocyte globulin in a murine BMT model.
Our model suggests operational tolerance since mice that received sirolimus for 15 or 31 days with PT-Cy maintained mixed chimerism, even as long as 11 months after discontinuing sirolimus therapy. No clinical signs of GVHD were observed in any mice. In contrast, donor cells were not detected in mice that received CSA with or without PT-Cy, engraftment remained very poor when CSA was delayed long enough for Cy to have been cleared, and recipient lymphocytes responded strongly to donor cells in MLR. Absence of such reactivity in the sirolimus +PT-Cy-treated mice would indicate operational tolerance to donor Ag permitting long-term engraftment. Skin graft results further confirmed functional tolerance in vivo, revealing that only C57BL/6 mice given sirolimus and PT-Cy to support BALB/c marrow engraftment tolerated secondary peripheral exposure to donor Ags.
Further, when CSA was given with sirolimus alone, mice supported donor grafts for 2–4 weeks, but they lost their grafts shortly thereafter. The regimen including CSA, sirolimus and PT-Cy was too toxic to evaluate whether CSA inhibits sirolimus- and PT-Cy-induced synergy. The few surviving mice that received the three agents displayed mixed chimerism, but it is possible that the CSA dose was not high enough to interfere with sirolimus. Other groups have suggested bortezomib as a synergistic alternative in combination with sirolimus, but these studies also rely upon infusion of previously tolerized splenic lymphocytes.42 Our data suggest that inhibiting T-cell responses with CSA does not allow mixed chimerism induction, while blocking mTOR promotes stable mixed chimerism without additional lymphocyte infusions.
In summary, our data define a novel strategy to promote mixed chimerism: deletion of alloreactive lymphocytes using PT-Cy followed by lymphocyte recovery under the cover of mTOR inhibition. As this approach does not inhibit TCR signaling, this regimen not only inhibits graft rejection but also promotes the generation of stable mixed chimerism. As such, we are hopeful that PT-Cy and mTOR inhibition can be used as a platform to develop a regimen supporting haploidentical HSCT for patients with nonmalignant hematologic diseases.
Supplementary Material
Acknowledgments
We thank Deborah Simon, Alex Agoreyo, Celinia Ondeck, Assefa Davis and all other Building 10A staff at the NIH for animal caretaking and procedural support, and Dr Alan Remaley for laboratory support.
Footnotes
Supplementary Information accompanies this paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)
References
- 1.Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–2756. doi: 10.1182/blood-2007-03-079665. [DOI] [PubMed] [Google Scholar]
- 2.Vermylen C, Cornu G, Ferster A, Brichard B, Ninane J, Ferrant A, et al. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplant. 1998;22:1–6. doi: 10.1038/sj.bmt.1701291. [DOI] [PubMed] [Google Scholar]
- 3.Walters MC, Patience M, Leisenring W, Eckman JR, Scott JP, Mentzer WC, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 4.Walters MC, Patience M, Leisenring W, Rogers ZR, Aquino VM, Buchanan GR, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7:665–673. doi: 10.1053/bbmt.2001.v7.pm11787529. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–2317. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh MM, Fitzhugh CD, Tisdale JF. Allogeneic hematopoietic stem cell transplantation for sickle cell disease: the time is now. Blood. 2011;118:1197–1207. doi: 10.1182/blood-2011-01-332510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durakovic N, Radojcic V, Powell J, Luznik L. Rapamycin promotes emergence of IL-10-secreting donor lymphocyte infusion-derived T cells without compromising their graft-versus-leukemia reactivity. Transplantation. 2007;83:631–640. doi: 10.1097/01.tp.0000256158.84418.ff. [DOI] [PubMed] [Google Scholar]
- 8.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4 + T cells, but enrich for antigen-specific Foxp3 + T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 9.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 10.Baan CC, van der Mast BJ, Klepper M, Mol WM, Peeters AM, Korevaar SS, et al. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation. 2005;80:110–117. doi: 10.1097/01.tp.0000164142.98167.4b. [DOI] [PubMed] [Google Scholar]
- 11.Powell JD, Fitzhugh C, Kang EM, Hsieh M, Schwartz RH, Tisdale JF. Low-dose radiation plus rapamycin promotes long-term bone marrow chimerism. Transplantation. 2005;80:1541–1545. doi: 10.1097/01.tp.0000185299.72295.90. [DOI] [PubMed] [Google Scholar]
- 12.Jager MD, Liu JY, Timrott KF, Popp FC, Stoeltzing O, Lang SA, et al. Sirolimus promotes tolerance for donor and recipient antigens after MHC class II disparate bone marrow transplantation in rats. Exp Hematol. 2007;35:164–170. doi: 10.1016/j.exphem.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Zhu Z, Huang Y, Bozulic LD, Hussain LR, Yan J, et al. Innate and adaptive immune responses are tolerized in chimeras prepared with nonmyeloablative conditioning. Transplantation. 2012;93:469–476. doi: 10.1097/TP.0b013e318242bddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kean LS, Adams AB, Strobert E, Hendrix R, Gangappa S, Jones TR, et al. Induction of chimerism in rhesus macaques through stem cell transplant and costimulation blockade-based immunosuppression. Am J Transplant. 2007;7:320–335. doi: 10.1111/j.1600-6143.2006.01622.x. [DOI] [PubMed] [Google Scholar]
- 15.Luznik L, Jones RJ, Fuchs EJ. High-dose cyclophosphamide for graft-versus-host disease prevention. Curr Opin Hematol. 2010;17:493–499. doi: 10.1097/MOH.0b013e32833eaf1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodsky RA, Luznik L, Bolanos-Meade J, Leffell MS, Jones RJ, Fuchs EJ. Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant. 2008;42:523–527. doi: 10.1038/bmt.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–3464. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 19.Colson YL, Wren SM, Schuchert MJ, Patrene KD, Johnson PC, Boggs SS, et al. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J Immunol. 1995;155:4179–4188. [PubMed] [Google Scholar]
- 20.Colson YL, Li H, Boggs SS, Patrene KD, Johnson PC, Ildstad ST. Durable mixed allogeneic chimerism and tolerance by a nonlethal radiation-based cytoreductive approach. J Immunol. 1996;157:2820–2829. [PubMed] [Google Scholar]
- 21.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8:131–138. doi: 10.1053/bbmt.2002.v8.pm11939602. [DOI] [PubMed] [Google Scholar]
- 22.Mayumi H, Umesue M, Nomoto K. Cyclophosphamide-induced immunological tolerance: an overview. Immunobiology. 1996;195:129–139. doi: 10.1016/S0171-2985(96)80033-7. [DOI] [PubMed] [Google Scholar]
- 23.Mayumi H, Himeno K, Tanaka K, Tokuda N, Fan JL, Nomoto K. Drug-induced tolerance to allografts in mice. XII. The relationships between tolerance, chimerism, and graft-versus-host disease. Transplantation. 1987;44:286–290. doi: 10.1097/00007890-198708000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Podder H, Stepkowski SM, Napoli KL, Clark J, Verani RR, Chou TC, et al. Pharmacokinetic interactions augment toxicities of sirolimus/cyclosporine combinations. J Am Soc Nephrol. 2001;12:1059–1071. doi: 10.1681/ASN.V1251059. [DOI] [PubMed] [Google Scholar]
- 25.Patel SJ, Elliott EN, Knight RJ, Gaber LW, Gaber AO. Considerations in sirolimus use in the early and late post-transplant periods. Expert opinion on drug safety. 2009;8:421–434. doi: 10.1517/14740330903037156. [DOI] [PubMed] [Google Scholar]
- 26.Brook NR, Waller JR, Bicknell GR, Nicholson ML. Cyclosporine and rapamycin act in a synergistic and dose-dependent manner in a model of immunosuppressant-induced kidney damage. Transplant Proc. 2005;37:837–838. doi: 10.1016/j.transproceed.2004.12.147. [DOI] [PubMed] [Google Scholar]
- 27.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coenen JJ, Koenen HJ, van Rijssen E, Kasran A, Boon L, Hilbrands LB, et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4 + CD25 + FoxP3 + T cells. Bone Marrow Transplant. 2007;39:537–545. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 29.Blazar BR, Taylor PA, Sehgal SN, Vallera DA. Rapamycin, a potent inhibitor of T-cell function, prevents graft rejection in murine recipients of allogeneic T-cell-depleted donor marrow. Blood. 1994;83:600–609. [PubMed] [Google Scholar]
- 30.Blazar BR, Taylor PA, Snover DC, Sehgal SN, Vallera DA. Murine recipients of fully mismatched donor marrow are protected from lethal graft-versus-host disease by the in vivo administration of rapamycin but develop an autoimmune-like syndrome. J Immunol. 1993;151:5726–5741. [PubMed] [Google Scholar]
- 31.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sehgal S, Vallera DA. In vivo inhibition of cytokine responsiveness and graft-versus-host disease mortality by rapamycin leads to a clinical–pathological syndrome discrete from that observed with cyclosporin A. Blood. 1996;87:4001–4009. [PubMed] [Google Scholar]
- 32.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4 + CD25 +FoxP3 + regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 34.Qu Y, Zhang B, Zhao L, Liu G, Ma H, Rao E, et al. The effect of immunosuppressive drug rapamycin on regulatory CD4 +CD25 +Foxp3 +T cells in mice. Transpl Immunol. 2007;17:153–161. doi: 10.1016/j.trim.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4( +)CD25( +) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 36.Pilat N, Klaus C, Gattringer M, Jaeckel E, Wrba F, Golshayan D, et al. Therapeutic efficacy of polyclonal tregs does not require rapamycin in a low-dose irradiation bone marrow transplantation model. Transplantation. 2011;92:280–288. doi: 10.1097/TP.0b013e3182241133. [DOI] [PubMed] [Google Scholar]
- 37.Hale DA, Gottschalk R, Fukuzaki T, Wood ML, Maki T, Monaco AP. Extended skin allo- and xenograft survival in mice treated with rapamycin, antilymphocyte serum, and donor-specific bone marrow transfusion. Transplant Proc. 1996;28:3269. [PubMed] [Google Scholar]
- 38.Hale DA, Gottschalk R, Fukuzaki T, Wood ML, Maki T, Monaco AP. Superiority of sirolimus (rapamycin) over cyclosporine in augmenting allograft and xenograft survival in mice treated with antilymphocyte serum and donor-specific bone marrow. Transplantation. 1997;63:359–364. doi: 10.1097/00007890-199702150-00005. [DOI] [PubMed] [Google Scholar]
- 39.Hale DA, Gottschalk R, Umemura A, Maki T, Monaco AP. Establishment of stable multilineage hematopoietic chimerism and donor- specific tolerance without irradiation. Transplantation. 2000;69:1242–1251. doi: 10.1097/00007890-200004150-00008. [DOI] [PubMed] [Google Scholar]
- 40.Prigozhina TB, Elkin G, Khitrin S, Slavin S. Prevention of acute graft-vs-host disease by a single low-dose cyclophosphamide injection following allogeneic bone marrow transplantation. Exp Hematol. 2008;36:1750–1759. doi: 10.1016/j.exphem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19:117–122. doi: 10.1016/j.bbmt.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Caballero-Velazquez T, Sanchez-Abarca LI, Gutierrez-Cosio S, Blanco B, Calderon C, Herrero C, et al. The novel combination of sirolimus and bortezomib prevents graft-versus-host disease but maintains the graft-versus-leukemia effect after allogeneic transplantation. Haematologica. 2012;97:1329–1337. doi: 10.3324/haematol.2011.058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.