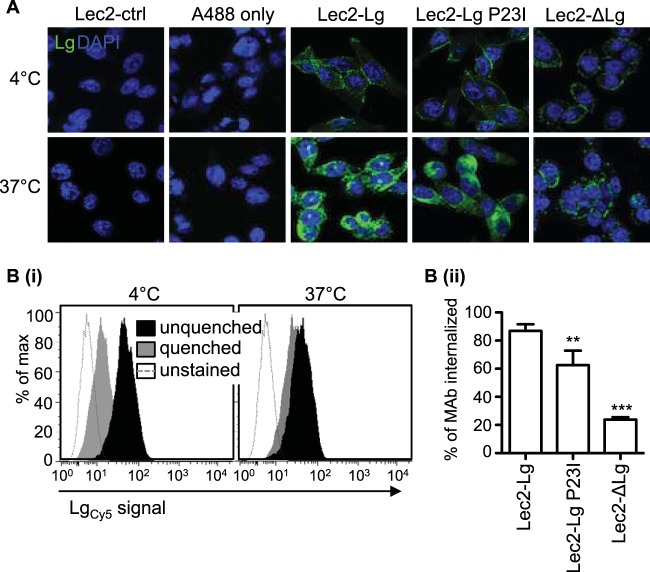
FIG 5.
Deletion of the intracellular domain of langerin results in an endocytosis-defective mutant, whereas the P23I mutation results in less significant impairment in endocytic activity. Lec2-Lg, Lec2-Lg P23I, Lec2-ΔLg, and Lec2-ctrl cells were incubated with a mouse MAb specific for human langerin or with Alexa Fluor 488 donkey anti-mouse IgG alone (A488 only) on Lec2-Lg cells at 4°C or 37°C for 30 min. (A) After this time, cells were fixed, permeabilized, and stained with Alexa Fluor 488 donkey anti-mouse IgG to amplify the fluorescent signal associated with antilangerin MAb bound to ligand. Representative images show surface and cytoplasmic staining of langerin in a single plane. (B) Reduced endocytic capacity of langerin expressed by Lec2-ΔLg cells was confirmed using a flow cytometry-based assay. Cells were incubated with FIPCy5-labeled antilangerin MAb (LgCy5) for 30 min on ice, washed, and then placed on ice or at 37°C for 30 min. After incubation, cells were washed and surface-bound MAb was quenched using a DNA quencher probe as described in Materials and Methods. (i) Representative histograms show binding of labeled MAb to Lec2-Lg cells after incubation at either 4°C or 37°C, in the presence or absence of quenching. (ii) Data were used to calculate the percentage of antilangerin MAb internalized by each cell line at 37°C. Data show the mean (±1 SD) from triplicate samples and were analyzed by one-way ANOVA with Tukey's post hoc analysis. **, P < 0.01; ***, P < 0.001.