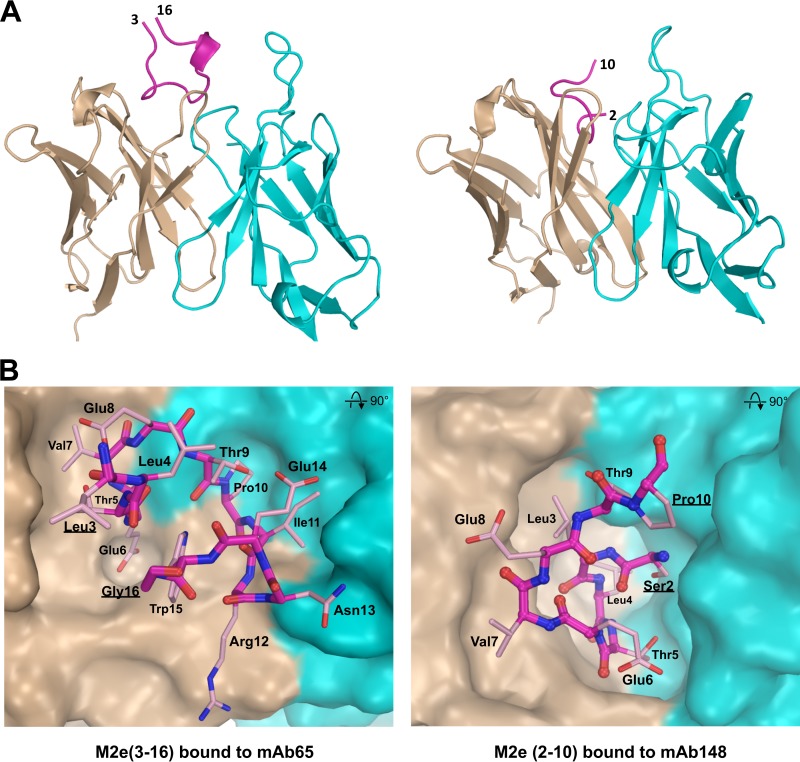
FIG 3.
Comparison of M2e bound to MAb65 and MAb148. (A) The conformation of M2e peptide bound by mAb65 (left) is entirely different from M2e bound by MAb148 (right). M2e peptide backbones are shown as purple lines. The 310 helix in M2e from Ile11 to Trp15 in the M2e-mAb65 complex is shown as a short ribbon, and the terminal residues of M2e are labeled. Antibody variable heavy and light chains are in brown and blue, respectively. (B) M2e has a β-turn from Thr5 to Glu8 and is folded in a U-shaped conformation with central Trp15 positioning when bound to MAb 65 (left) (PDB ID 4N8C [6]). M2e has an N-terminal β-turn involving Ser2 to Thr5 when M2e interacts with MAb 148 (right). The surfaces of heavy and light chains are in brown and blue, respectively.