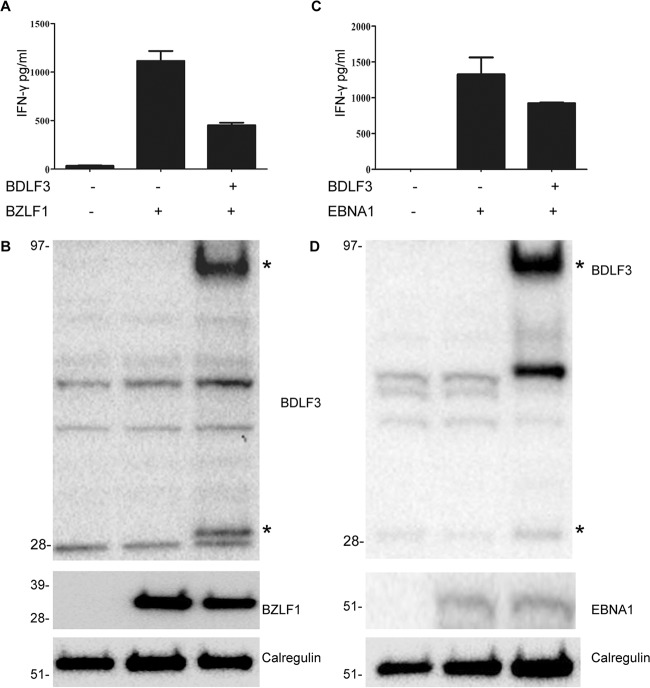
FIG 4.
BDLF3 can inhibit EBV-specific CD8+ and CD4+ T cell recognition. MJS cells were cotransfected with the p509 plasmid (BZLF1 expression vector) together with control-GFP or BDLF3-GFP. At 24 h posttransfection, the MJS cells were cocultured with effector T cells, i.e., a BZLF1 (RAK)-specific CD8+ T cell clone, for a further 18 h, and the supernatants were tested for the release of IFN-γ as a measure of T cell recognition. All results are expressed as amounts of IFN-γ release (pg/ml), and error bars indicate standard deviations for triplicate cultures. (B) Total cell lysates were generated from the above transfections and analyzed by Western blotting using antibodies specific for BDLF3, BZLF1, and calregulin (loading control). The asterisks adjacent to the BDLF3 blot indicate monomeric and trimeric forms of BDLF3. (C) MJS-DR51 cells were first transfected with EBNA1ΔNLS, allowed to recover in culture overnight, and then divided into two groups and transfected with either BDLF3-NGFR or control-NGFR. After another 24 h, NGFR+ BDLF3+ or control NGFR+ cells were sorted with magnetic beads and used as targets for HLA-DR51-restricted, EBNA1 (SNP)-specific CD4+ T cell clones. Recognition was measured as the amount of IFN-γ release (pg/ml) by T cell clones. Error bars represent standard deviations of the means for triplicate assay replicates. Results are representative of three independent experiments. (D) Total cell lysates were generated from the above transfections and analyzed by Western blotting using antibodies specific for BDLF3, EBNA1, and calregulin (loading control).