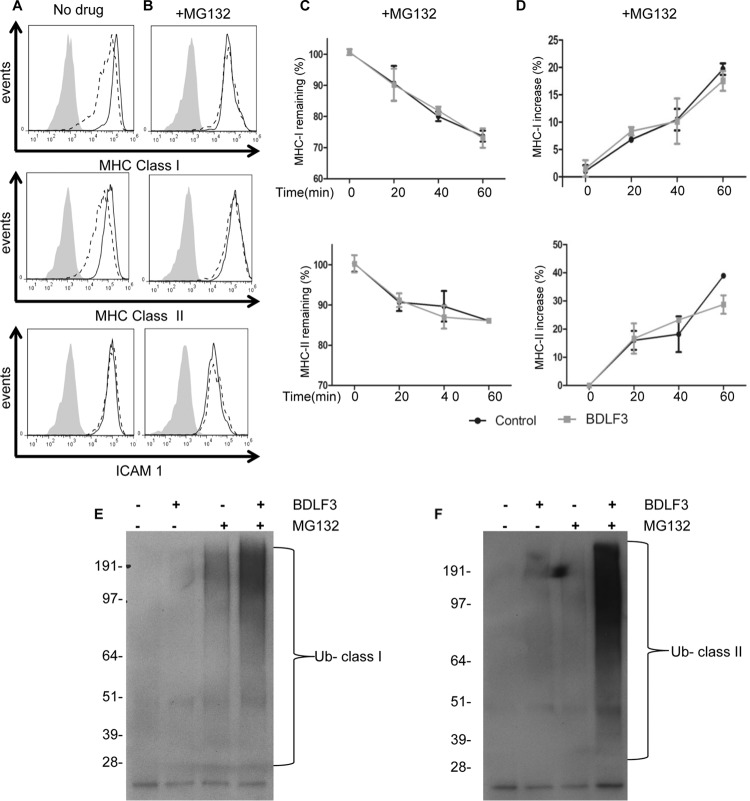
FIG 7.
Treatment of BDLF3-expressing cells with a proteasome inhibitor prevents downregulation of MHC class I and class II. MJS cells were transiently transfected with the BDLF3-GFP or control-GFP plasmid and then incubated in normal medium (A) or MG132 (5 μM)-supplemented medium (B). At 24 h posttransfection, two-color flow cytometry was used to measure surface MHC class I (upper histograms), surface MHC class II (middle histograms), and surface ICAM1 (lower histograms) in GFP+ populations of control-GFP-transfected cells (solid lines) and BDLF3-GFP-transfected cells (dashed lines). The gray histograms denote background staining obtained with an isotype control antibody. Results are representative of repeat experiments (n > 4). (C and D) MJS cells were transiently transfected with the BDLF3-GFP or control-GFP plasmid and then were incubated with MG132 (5 μM). Following drug treatment, the rates of internalization (C) of MHC-I (top panel) and MHC-II (bottom panel) and the rates of appearance (D) of MHC-I (top panel) and MHC-II (bottom panel) were measured using the same methods as those described in the legend to Fig. 6. (E and F) MJS cells were transfected with a ubiquitin expression plasmid plus either the control-NGFR or BDLF3-NGFR plasmid. The transfected cells were then divided into two groups and incubated in normal medium or medium supplemented with MG132. At 24 h posttransfection, NGFR+ BDLF3+ or control NGFR+ cells were sorted with magnetic beads, and surface MHC class I (C) or MHC class II (D) molecules were immunoprecipitated, eluted, and then immunoblotted using an anti-ubiquitin (Ub) antibody (P4D1).