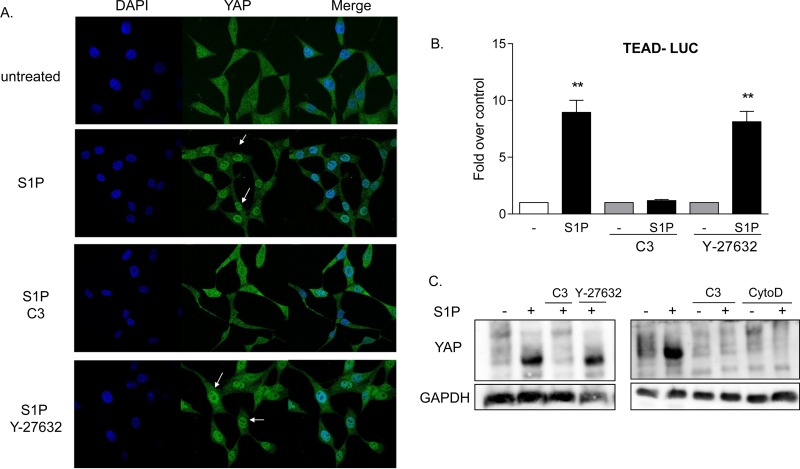
FIG 3.
YAP is activated by S1P through RhoA signaling. (A) Twenty-four-hour serum-starved glioblastoma cells were pretreated with 1 μg/ml C3 exoenzyme (24 h) or 10 μM Y-27632 (1 h). Cells were then stimulated for 1 h with 0.3 μM S1P. YAP subcellular localization was determined by immunofluorescence staining for endogenous YAP (green) along with DAPI staining to show nuclei (blue). (B) Cells were transfected with a TEAD promoter-driven firefly luciferase reporter construct (TEAD-Luc) for 24 h. Cells were serum starved for 24 h prior to being pretreated with 1 μg/ml C3 exoenzyme (24 h) or 10 μM Y-27632 (1 h), stimulated with 0.3 μM S1P for 8 h, and assayed for luciferase expression. Data shown are normalized to Renilla luciferase activity and expressed as fold changes over the control level. **, P < 0.01 versus control (n = 6). (C) Twenty-four-hour serum-starved glioblastoma cells were pretreated with 1 μg/ml C3 exoenzyme (24 h), 10 μM Y-27632 (1 h), or 10 μM cytochalasin D (CytoD) (1 h). Cells were then stimulated for 1 h with 0.3 μM S1P, and YAP phosphorylation was determined by Phos-tag immunoblotting for YAP. Note that the downshift representing dephosphorylation is also accompanied by greater immunoreactivity, as seen by others (27, 29).