FIG 7.
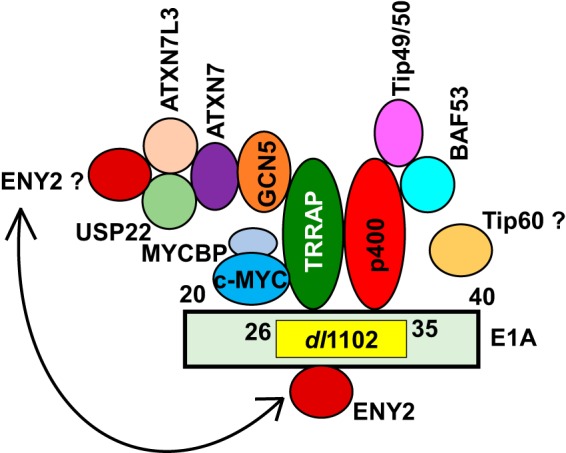
Interaction of cellular proteins with the transforming domain of E1A. The interactions of TRRAP, p400, and GCN5 with the E1A domain spanning residues 26 to 35 were previously known. These interactions were not seen with the mutant dl1102. Our present results revealed the interactions of other proteins shown in the figure via TRRAP and p400. The interaction of Tip60 (indicated by the question mark) was not detected in our LC-MS or by E1A co-IP experiments. Our results suggest that ENY2 may interact with E1A independently of TRRAP. Since ENY2 is an integral part of the USP22 DUB complex, we postulate that ENY2 may additionally interact with E1A via the USP22 DUB complex.
