Abstract
The Arctic Investigations Program (AIP) began surveillance for invasive group A streptococcal (GAS) infections in Alaska in 2000 as part of the invasive bacterial diseases population-based laboratory surveillance program. Between 2001 and 2013, there were 516 cases of GAS infection reported, for an overall annual incidence of 5.8 cases per 100,000 persons with 56 deaths (case fatality rate, 10.7%). Of the 516 confirmed cases of invasive GAS infection, 422 (82%) had isolates available for laboratory analysis. All isolates were susceptible to penicillin, cefotaxime, and levofloxacin. Resistance to tetracycline, erythromycin, and clindamycin was seen in 11% (n = 8), 5.8% (n = 20), and 1.2% (n = 4) of the isolates, respectively. A total of 51 emm types were identified, of which emm1 (11.1%) was the most prevalent, followed by emm82 (8.8%), emm49 (7.8%), emm12 and emm3 (6.6% each), emm89 (6.2%), emm108 (5.5%), emm28 (4.7%), emm92 (4%), and emm41 (3.8%). The five most common emm types accounted for 41% of isolates. The emm types in the proposed 26-valent and 30-valent vaccines accounted for 56% and 78% of all cases, respectively. GAS remains an important cause of invasive bacterial disease in Alaska. Continued surveillance of GAS infections will help improve understanding of the epidemiology of invasive disease, with an impact on disease control, notification of outbreaks, and vaccine development.
INTRODUCTION
Streptococcus pyogenes (group A streptococci [GAS]) is an exclusively human pathogen that is usually transmitted through contact with respiratory secretions from an infected person. GAS is most commonly associated with pharyngitis and skin infections but can also cause more serious invasive infections, including puerperal sepsis, bacteremia, pneumonia, meningitis, necrotizing fasciitis (NF), and streptococcal toxic shock syndrome (STSS) (1). It can also lead to serious nonsuppurative sequelae, such as acute rheumatic fever and rheumatic heart disease, especially in developing countries (1–3). Since the mid-1980s, a surge in the incidence and severity of GAS infections has been documented worldwide, resulting in significant morbidity and mortality (4–8). In the United States from 2000 to 2005, the annual average incidence of invasive GAS disease was 3.5 cases per 100,000 persons, with 735 deaths (case fatality rate, 13.7%) (9). Persons ≥65 years of age had the highest incidence (9.4 cases per 100,000 persons), followed by children <1 year of age (5.3 cases per 100,000 persons). The case fatality rate (22.8%) was also highest among the elderly.
Strain characterization of GAS was traditionally based on serological identification of the M protein, which is a major surface protein and an important GAS virulence factor. Classical serologic M typing in many laboratories has been replaced by emm typing, which in almost all cases predicts the classical M serotype (10, 11). To date, >200 different emm types have been reported. In most countries, emm types 1, 3, 12, and 28 have traditionally been associated with invasive GAS disease (9, 12–22).
Clinical management of invasive GAS infections centers on accurate diagnosis early in the course of disease and timely, appropriate use of antibiotics. Few effective prevention strategies exist. Efforts at disease prevention have been focused on vaccine development. However, development of a GAS vaccine has been challenging because of the vast number of emm types and because of concerns of possible induction of antibodies that cross-react with epitopes in the GAS M protein and human brain, joint, and cardiac tissues (23–25). Molecular techniques have allowed the development of multivalent M protein-based vaccines that contain protective epitopes and exclude potentially harmful tissue cross-reactive epitopes (25–28). A 26-valent vaccine containing M peptides from serotypes of GAS representing the vast majority of infections in the United States was reported as safe and immunogenic in adult volunteers (29). More recently, Dale et al. constructed a 30-valent vaccine composed of M peptides representing the majority of infections in the United States and Europe (30). In animal studies, this vaccine evoked bactericidal antibodies against all of the vaccine serotypes. This vaccine was also found to elicit significant bactericidal activity against a number of laboratory strains of GAS that were nonvaccine serotypes, suggesting increased efficacy beyond the serotypes in the vaccine (31). With this progress, baseline data on the burden of disease are critical in order to evaluate the potential utility of these candidate GAS vaccines.
The Arctic Investigations Program (AIP) began surveillance for invasive GAS infections in Alaska in 2000 as part of the invasive bacterial diseases population-based laboratory surveillance program. Here, we report the epidemiologic characteristics of invasive GAS infection in Alaska over 13 years of surveillance (2001 to 2013).
MATERIALS AND METHODS
Population studied.
Alaska's population of 710,231 (2010 U.S. census) includes 142,000 (20%) Alaska Native (AN) and American Indian peoples, 7,100 of whom are younger than 2 years of age. Sixty-five percent of Alaska Native peoples live in rural communities, many of which are isolated villages with populations ranging from 50 to 1,000 persons. Health care for the AN population is provided through a statewide tribally operated health delivery system which includes community health practitioners at the village level, primary care providers in regional hub communities, and a referral hospital in Anchorage.
Case definition and patient characteristics.
AIP conducts population-based statewide surveillance for invasive infections due to GAS and other bacterial pathogens of public health concern in Alaska. We reviewed reports of invasive GAS cases occurring from 1 January 2001 through 31 December 2013. A case of invasive GAS infection was defined as the isolation of group A Streptococcus from a sterile site (e.g., blood, cerebrospinal fluid, pleural fluid, peritoneal fluid, pericardial fluid, surgical aspirate, bone, joint fluid, or internal body site) or from a wound culture accompanied by necrotizing fasciitis (NF) or streptococcal toxic shock syndrome (STSS) in a resident of Alaska. Demographic and clinical data on cases were collected by reviewing medical records and electronic documents. We did not assess sequelae such as acute rheumatic fever, rheumatic heart disease, or poststreptcoccal glomerulonephritis.
Bacterial isolates.
Isolates of group A streptococci are received at the AIP laboratory in Anchorage, AK, from 23 regional hospital laboratories processing sterile site isolates in the state. GAS isolates were confirmed by β-hemolysis on sheep blood agar, Lancefield antigen grouping using a commercially available agglutination test kit (Phadebact Strep A kit), and the pyrrolidonyl-arylamidase test (PYR 50 test kit; Remel Inc., Lenexa, KS).
emm typing.
DNA extracts were prepared as follows: bacterial cells were resuspended in 300 μl 0.85% NaCl, heated at 70°C for 15 min, and centrifuged, and pellets were incubated in 50 μl 10 mM Tris and 1 mM EDTA (pH 8.0) with 2 μl hyaluronidase (30 mg/ml) and 10 μl mutanolysin (3,000 units/ml) for 30 min at 37°C, heated at 100°C for 10 min, and frozen at −30°C as modified from the protocol at http://www.cdc.gov/streplab/protocol-emm-type.html. The emm gene was amplified and sequenced according to the Centers for Disease Control and Prevention protocol (http://www2a.cdc.gov/ncidod/biotech/strepblast.asp).Sequence analysis was performed by a BLAST search on the Centers for Disease Control and Prevention streptococcal emm sequence database (http://www2a.cdc.gov/ncidod/biotech/strepblast.asp) to designate emm sequence type.
Antimicrobial susceptibility testing.
Susceptibility testing was performed on all GAS isolates beginning in 2004 using the standard broth microdilution method as described by the Clinical and Laboratory Standards Institute (CLSI), for penicillin, erythromycin, tetracycline, levofloxacin, cefotaxime, and clindamycin (32). The MIC was determined to be the lowest concentration of antibiotic that inhibited growth. The MIC results were interpreted according to the 2011 CLSI criteria which included the following breakpoints: penicillin resistance, >0.12 μg/ml; tetracycline resistance, >8.0 μg/ml; erythromycin resistance, >1.0 μg/ml; levofloxacin resistance, >8.0 μg/ml; cefotaxime resistance, >0.5 μg/ml; and clindamycin resistance, >1.0 μg/ml (32).
Statistical analysis.
Incidence calculations are expressed as the number of cases per 100,000 population. Population estimates were obtained from the State of Alaska Department of Labor and Work Force Development (33). The trend in GAS rates over time was tested by use of Poisson regression. The Poisson test of GAS rates between AN persons and non-AN persons was age adjusted. We compared case fatality rates by use of the likelihood ratio chi-square test and developed a multivariate model using logistic regression. Simpson's diversity index (D) was used to summarize the diversity of GAS emm types (34). The higher the index, the greater the diversity of emm types. We selected Simpson's index because it gives more weight to the relatively common emm types and because it is less sensitive to changes in sample size (35). All P values are two-sided, and a value of <0.05 was considered statistically significant. When necessitated by sample size, an exact P value was reported.
RESULTS
Descriptive epidemiology, incidence rates, and clinical syndrome data.
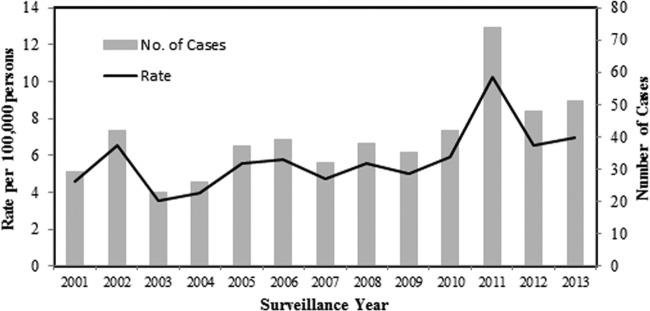
Over the 13-year study period (January 2001 through December 2013), 516 cases of invasive GAS disease were reported to AIP; 285 (55%) of the patients were male. The median age of case patients was 47.3 years. The overall incidence rate over the 13-year study period was 5.8 cases per 100,000 persons (Table 1). The rate during the period 2010 to 2013 of 7.4 cases per 100,000 persons was significantly higher (P < 0.001) than the rate (4.8 cases per 100,000 persons) during the first time period (2001 to 2005). Much but not all of this difference can be attributed to the increase in the number of cases in 2011 (P = 0.02 with 2011 data excluded), and it reflects a trend toward an increase in number of cases in 2012 and 2013 compared to previous years (Fig. 1). Rates of GAS disease were 14.8 cases per 100,000 persons among children <2 years of age and 15.7 cases per 100,000 among persons >65 years of age. Ethnicity data were available for all cases; 237 (46%) occurred in AN persons (Table 1). Rates of invasive GAS disease among AN persons compared to non-AN persons were 13.7 versus 3.9 cases per 100,000 persons (P < 0.0001), respectively. Rates of disease among AN children <2 years of age compared to non-AN children of the same age were 39.9 versus 4.2 cases per 100,000 persons (P < 0.0001), respectively. Cases occurred in all regions of the state.
TABLE 1.
Rates of invasive group A streptococcal disease in Alaska, 2001 to 2013
| Factor | Group | No. of cases | Rate (per 100,000 persons) |
|---|---|---|---|
| Yr (all ages) | 2001–2013 | 516 | 5.8 |
| 2001–2005 | 157 | 4.8 | |
| 2006–2009 | 144 | 5.3 | |
| 2010–2013 | 215 | 7.4 | |
| Age class (yr) | <2 | 40 | 14.8 |
| 2–4 | 14 | 3.3 | |
| 5–17 | 32 | 1.8 | |
| 18–44 | 148 | 4.3 | |
| 45–64 | 182 | 7.7 | |
| 65+ | 100 | 15.7 | |
| Sex (all ages) | Male | 285 | 6.2 |
| Female | 231 | 5.4 | |
| Clinical infectiona,b | Endocarditis | 15 | 0.17 |
| Empyema | 22 | 0.25 | |
| Toxic shock syndrome | 16 | 0.18 | |
| Pneumonia | 101 | 1.14 | |
| Necrotizing fasciitis | 41 | 0.46 | |
| Septic arthritis | 44 | 0.49 | |
| Cellulitis | 217 | 2.44 | |
| Bacteremia | 121 | 1.36 | |
| Severity measures | Hospitalizations | 443 | 5.0 |
| Deaths | 56 | 0.6 | |
| Race | Alaska Native persons | 237 | 13.7c |
| Non-AN persons | 279 | 3.9c |
A case was included in the rate for a specific clinical infection if it appeared alone or in combination with other clinical infections; infections are not mutually exclusive. “Case” is defined as bacteremic only if no other infection was present.
When cases are placed in only one infection, prioritized according to their display in table (top to bottom), the rates are as follows: endocarditis, 0.17; empyema, 0.25; toxic shock syndrome, 0.16; pneumonia, 0.84; necrotizing fasciitis, 0.38; septic arthritis, 0.39; cellulitis, 1.73; and bacteremia, 1.36.
Age-standardized rates: 14.8 and 3.8.
FIG 1.
Incidence rates and numbers of cases of invasive group A streptococcal disease by year in Alaska, 2001 to 2013.
The most common clinical manifestations were cellulitis (42%, n = 217), bacteremia (23%, n = 121), and pneumonia (19.6%, n = 101). Patients with NF and STSS accounted for 8% (n = 41) and 3% (n = 16) of cases, respectively (Table 1). Eighty-six percent (443/516) of patients were hospitalized. The median length of hospitalization was 8.0 days for all cases; 15.0 for cases of necrotizing fasciitis and 16.0 days for cases of TSS. Among the 516 case patients for whom outcome data were reported, 56 died (case fatality rate of 10.7%); four deaths were among cases with NF, and two deaths occurred in individuals diagnosed with STSS.
Risk factors for death.
Risk for death increased with age (P = 0.001) and was highest among those ≥65 years of age (20%, n = 20) (Table 2). We did not find any difference in risk of death according to clinical presentation, with the exception that patients diagnosed with cellulitis were less likely to die than those without this diagnosis (P = 0.002). Risk for death did vary according to the emm type responsible for the infection. The highest risk was associated with emm types 1, 3, and 12, and the difference was statistically significant (P < 0.0001).
TABLE 2.
Risk factors for death among cases of invasive group A streptococcal disease in Alaska, 2001 to 2013
| Risk factor | Group | % case fatality (ratio) | Rate ratioa | P value |
|---|---|---|---|---|
| Period | 2001–2005 | 10.2 (16/157) | Ref | 0.89 |
| 2006–2009 | 10.5 (15/143) | 1.0 | ||
| 2010–2013 | 11.6 (25/215) | 1.1 | ||
| Sex | Female | 11.7 (27/231) | 1.1 | 0.59 |
| Male | 10.2 (29/284) | Ref | ||
| Age | ≤64 years | 8.7 (36/415) | Ref | 0.001b |
| 65+ years | 20.0 (20/100) | 2.3 | ||
| Ethnicity | Alaska Native | 8.9 (21/237) | 0.7 | 0.18 |
| Non-Alaska Native | 12.6 (35/278) | Ref | ||
| emm type | 1, 3, and 12 | 24.5 (25/102) | 3.4 | <0.0001b |
| All others | 7.2 (23/319) | Ref | ||
| Endocarditis | Yes | 35.7 (5/14) | 2.5 | 0.01 |
| No | 10.2 (51/501) | Ref | ||
| Empyema | Yes | 4.6 (1/22) | 0.4 | 0.49 |
| No | 11.2 (55/493) | Ref | ||
| Toxic shock syndrome | Yes | 12.5 (2/16) | 1.2 | 0.70 |
| No | 10.8 (54/499) | Ref | ||
| Pneumonia | Yes | 14.9 (15/101) | 1.5 | 0.15 |
| No | 9.9 (41/413) | Ref | ||
| Necrotizing fasciitis | Yes | 10.0 (4/40) | 0.9 | 1.00 |
| No | 11.0 (52/475) | Ref | ||
| Septic arthritis | Yes | 4.6 (2/44) | 0.4 | 0.21 |
| No | 11.5 (54/471) | Ref | ||
| Cellulitis | Yes | 6.0 (13/217) | 0.4 | 0.002b |
| No | 14.4 (43/298) | Ref |
Ref, reference.
All remained significant in a multivariate logistic regression: age class (P = 0.007); emm type 1, 3, or 12 (P < 0.0001); and cellulitis (P = 0.007).
emm sequence types.
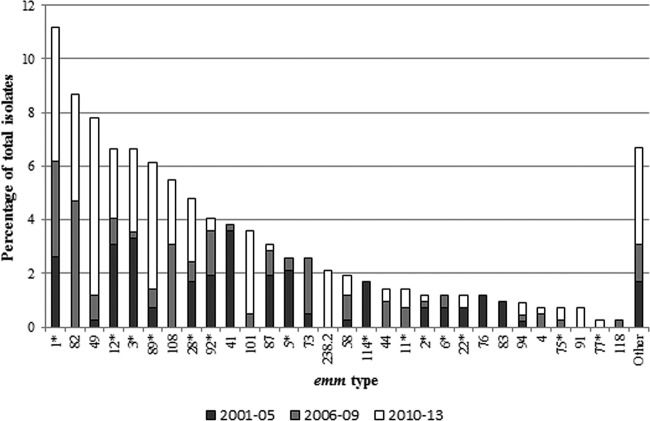
Of the 516 cases included in our study, isolates from 422 (82%) were available for evaluation. Among these 422 isolates, 51 different emm types were identified. The 10 most common emm types accounted for 65% (275/422) of isolates and included types 1, 82, 49, 12, 3, 89, 108, 28, 92, and 41 (Fig. 2). The distribution of emm types varied significantly over time (Table 3). The most common emm types seen during surveillance years 2001 to 2005 were types 41 (12%, n = 15), 3 (12%, n = 12), and 12 (11%, n = 13). During surveillance years 2006 to 2009, the most common emm types were 82 (18%, n = 20), 1 (13%, n = 15), and 108 (12%, n = 13). emm types 49 (15%, n = 28), 1 (11%, n = 21), and 89 (11%, n = 20) were the most common types found during surveillance years 2010 to 2013. The significant difference in emm types (P < 0.0001) between surveillance periods was accounted for by increases in emm types 49, 82, 89, and 108 and decreases in emm types 3, 12, and 41 (Fig. 2). The significant difference in emm types (P < 0.0001) by race/ethnicity was accounted for by a higher prevalence of emm types 49, 82, 87, 92, and 101 in AN persons and a higher prevalence of emm types 1, 3, 12, 28, and 89 in non-AN persons. The distribution of emm types did not significantly differ on the basis of sex, age (<45 years versus ≥45 years), residence (urban versus rural), or clinical presentation (cellulitis versus other).
FIG 2.
Distribution of emm types among invasive group A streptococcal isolates in Alaska by time period, 2001 to 2006, 2006 to 2009, and 2010 to 2013. Asterisks indicate emm types present in both the 26-valent and 30-valent vaccines. emm types present in the 30-valent but not in the 26-valent vaccine include types 82, 49, 87, 73, 58, 44, 83, 4, and 118. emm types contained in the 26-valent but not in the 30-valent include types 76, 94, and 101. emm types that are not included in either vaccine are types 108, 41, and 1-2.3.
TABLE 3.
Comparison of emm type distribution among invasive group A streptococcal isolates in Alaska between 2001–2005, 2006–2009, and 2010–2013
| Descriptor | 2001–2005 (n = 121) | 2006–2009 (n = 113) | 2010–2013 (n = 188) |
|---|---|---|---|
| No. of emm types | 22 | 28 | 36 |
| Most common emm type (%) | 41 (12) | 82 (18) | 49 (15) |
| 3 (12) | 1 (13) | 1 (11) | |
| 12 (11) | 108 (12) | 89 (11) | |
| % of cases (top 5 emm types) | 51 (62/121) | 57 (64/113) | 53 (99/188) |
| % of cases (top 10 emm types) | 80 (97/121) | 74 (84/113) | 80 (150/188) |
| Simpson's diversity index | 0.92 | 0.90 | 0.93 |
| % covered by 26-valent vaccine | 72.7 (88/121) | 39.8 (45/113) | 55.3 (104/188) |
| % covered by 30-valent vaccine | 80 (97/121) | 79.6 (90/113) | 75.5 (142/188) |
| Most common emm type(s) not in either vaccine | 41 | 108, 9 | 108, 1-2.3, 91 |
With the possibility of two GAS vaccines coming onto the market, we evaluated the proportion of our invasive GAS cases that were preventable with use of these vaccines (Table 3; Fig. 2). During the early surveillance period (2001 to 2005), the 26-valent vaccine covered 73% (88/121) of cases, followed by 40% (45/113) of cases during surveillance years 2006 to 2009 and 55% (104/188) of cases during 2010 to 2013. The 30-valent vaccine would have covered 80% (97/121), 77% (90/113), and 74% (142/188) of cases in surveillance years 2001 to 2005, 2006 to 2009, and 2010 to 2013, respectively. Across the 13-year surveillance period, 56% (237/422) and 76% (321/422) of the isolates were emm types included in the 26-valent and 30-valent vaccines, respectively. The proportion of disease accounted for by emm types in the 26-valent vaccine was higher among adults ≥65 years of age (59%, n = 59) than children <5 years of age (50%, n = 27). Coverage with the 30-valent vaccine was similar (79% [n = 79] versus 84% [n = 45]). When broken down by clinical syndrome, 62% (21/34) of NF cases, 67% (8/12) of STSS cases, and 73% (35/48) of deaths were potentially preventable by the 26-valent vaccine. The proportions of NF and STSS cases potentially preventable by the 30-valent vaccine were 68% (23/34) and 75% (9/12), respectively, and at least 85% (41/48) of deaths were preventable by this vaccine. A diversity of emm types was detected in isolates from patients with the most severe forms of infection (NF and SSTS). Among the 34 cases of NF, 20 different emm types were observed; the most common types included 1 (n = 5), 12 (n = 4), and 89 (n = 3). Eight different emm types were observed among the 12 cases of STSS; the most common types included 1 (n = 4) and 3 (n = 2).
Antimicrobial susceptibility.
Of the 343 isolates tested, all were susceptible to penicillin, cefotaxime, and levofloxacin. Resistance to tetracycline, erythromycin, and clindamycin was seen in 11% (n = 38), 5.8% (n = 20), and 1.2% (n = 4) of the isolates, respectively (Table 4). The proportion of isolates resistant only to tetracycline varied over time. From 2004 to 2008, 20% (25/127) of isolates were resistant to tetracycline, compared to <1% (2/216) during surveillance years 2009 to 2013 (P < 0.001). The majority (81%) of the isolates resistant only to tetracycline during the early surveillance years were emm types 87 (n = 12), 108 (n = 6), and 49 (n = 4).
TABLE 4.
Distribution of emm types among antimicrobial resistant invasive group A streptococcal isolates in Alaska by time perioda
| Time period | No. of isolates | Tetr |
Eryr |
Tetr Eryr |
Eryr Cmr |
Tetr Eryr Cmr |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | emm type(s) (no. of isolates) | No. of isolates | emm type(s) (no. of isolates) | No. of isolates | emm type(s) (no. of isolates) | No. of isolates | emm type (no. of isolates) | No. of isolates | emm types (no. of isolates) | ||
| 2004–2005 | 48 | 9 | 41, 87 (8) | 0 | NA | 1 | 58 | 1 | 92 | 0 | NA |
| 2006–2009 | 112 | 16 | 1, 41, 49 (4), 87 (4), 108 (6) | 5 | 92 | 5 | 58 (4), 94 | 0 | NA | 0 | NA |
| 2010–2013 | 183 | 2 | 44, stG6.0 | 3 | 3, 75 (2) | 2 | 58 | 0 | NA | 3 | 11 (2), 94 |
Tet, tetracycline; Ery, erythromycin; Cm, clindamycin; NA, not applicable.
The majority (65%) of isolates resistant only to erythromycin were emm type 92 (n = 5) and 75 (n = 2). Nine isolates (2.6%) were resistant to both tetracycline and erythromycin and were represented by emm types 58 (n = 7) and 94 (n = 2). All emm types associated with tetracycline and erythromycin resistance in our study, except emm108, are contained in both the 26-valent and 30-valent vaccines.
DISCUSSION
Here, we report a comprehensive description of the epidemiology of group A streptococcal disease in the state of Alaska from 2001 to 2013, including information on emm type prevalence. With the reemergence of invasive GAS disease in the 1980s, there have been numerous reports published describing the burden of GAS disease in industrialized countries (7, 9, 13, 17–20, 36). In these reports, invasive GAS disease rates ranged from 1.5 to 5.8 cases/100,000 persons. In the United States, O'Loughlin et al. (9) reported a mean incidence of 3.5 cases/100,000 persons from 2000 to 2004 among the CDC's Active Bacterial Disease Surveillance network, which is within the range reported worldwide. However, a recent study by Stockmann et al. reported a mean annual incidence rate of 6.3 cases/100,000 persons among Utah residents, with the rate increasing from 3.5 cases/100,000 persons in 2002 to 9.8 cases/100,000 persons in 2010 (37). The overall annual incidence of invasive GAS disease in Alaska is slightly higher (5.8/100,000 population) than reported from CDC's Active Bacterial Disease Surveillance network (3.7/100,000; 2001 to 2013; http://www.cdc.gov/abcs/reports-findings/surv-reports.html). When we compared the incidence among Alaska Native persons (13.7/100,000) to that in non-AN persons (3.9/100,000), the rate was much higher among AN persons and similar to what has been reported for developing countries such as Fiji and for indigenous populations in Australia and New Zealand (38–40). In our study, the highest burden of disease was found among children <2 years of age (14.8 cases/100,000 persons) and adults >65 years of age (15.7 cases/100,000 persons). These rates are higher than what was reported for the general U.S. population from 2001 to 2013 (4.6 cases/100,000 in children <2 years old and 9.7 cases/100,000 in adults ≥65 years old; http://www.cdc.gov/abcs/reports-findings/surv-reports.html) and is most likely due to the higher rates of disease among Alaska Native persons.
The most prevalent clinical manifestation was cellulitis, comprising 42% of all invasive cases, which is similar to what has been reported in several countries in Europe and the United Kingdom (18, 41). Other studies from the United States have reported that the clinical syndromes associated with invasive GAS disease vary from year to year (8, 9, 37). The overall case fatality rate reported in our study was 10.7% which is similar to what has been reported by others. The highest case fatality rate in our study was associated with persons diagnosed with endocarditis (35.7%).
In this study, the five most common emm types were types 1, 3, 12, 49, and 82. Of these, emm types 1, 3, and 12 have been reported to be among the most prevalent types causing invasive disease worldwide (41). However, there are significant differences in emm type distribution by region and country, with a much greater diversity of strains in low-income settings. Using Simpson's index of diversity, which reflects the probability that two isolates selected at random from the same population will be of different emm types, we calculated a diversity index of 0.92 among our isolates. This was similar to what has been reported for other high-income settings but not as high as what has been reported for regions such as Africa and the Pacific, where the diversity index ranged from 0.97 to 0.98 (41).
We found considerable variation in the distribution of emm types between the three time periods analyzed. Variation in the relative prevalence of predominant emm types over time is not unique to our study, as others have also found significant variability (12–14, 18, 20, 36, 42). Such variability can potentially limit the usefulness of vaccines based on the M protein. We did note, however, that some emm types (types 1, 12, 28, 58, 89, and 92) in our study were seen in all or most surveillance years, while other emm types emerged for a limited time (types 5, 41, 73, 76, 83, and 114) and then declined. Notably, emm type 49 cases seemed to have higher mortality (4 deaths in 18 cases versus 4 deaths in 41 cases for non-emm49 cases); however, this did not reach statistical significance (P = 0.095). In other studies, emm type 49 strains have been associated with severe invasive infections and evasion of the host neutrophilic defenses (43, 44).
We investigated whether certain emm types are associated with particular clinical syndromes or mortality and found that mortality was significantly associated with emm types 1, 3, and 12, but we did not observe an association between emm type and severe disease (NF and STSS). A few studies have shown a positive correlation between emm type and disease severity (8, 14, 15, 17, 41). Among the 34 cases of NF in our study, 20 different emm types were observed, including five cases with emm1. Among the 12 cases of STSS, eight different emm types were found, including four cases with emm1. The relative importance of any individual emm type in disease severity is likely the result of a combination of multiple factors, such as the relative frequency of emm types circulating within a community, the degree of individual and community-level immunity to the circulating strains, and the invasiveness of these strains.
While there has been an apparent reemergence of invasive GAS disease in the last few decades, strategies for preventing morbidity and mortality are still limited. In this study, emm types contained in the proposed 26-valent vaccine accounted for 56% of all isolates, while the proposed 30-valent vaccine accounted for 78% of all isolates. However, there was considerable variability in the proportion of emm types included in both of these vaccines by year. Knowing how a vaccine would match the epidemiology of GAS emm types is useful for planning purposes. However, the effectiveness of a GAS vaccine in Alaska would also depend greatly on the specific recommendations for age and risk groups, vaccine uptake, and efficacy of the vaccine against invasive disease.
Recently, a functional classification based on 48 emm clusters containing closely related M proteins that share binding and structural properties was proposed (45). In this classification system, M proteins included in the same emm cluster demonstrate, by definition, an average pairwise identity of >70% and share similar binding properties, and therefore they potentially provide immunologic cross-protection. While the authors of that study do not suggest replacing emm typing with emm cluster typing, they suggest that this new classification system will help facilitate the design of studies to investigate GAS molecular epidemiology and can support vaccine design and evaluation. Using this classification system, we observed that the 51 emm types identified in our study, belonged to 16 emm clusters (data not shown). Seven emm clusters were responsible for the majority (90%) of cases.
All isolates in this study were susceptible to penicillin, cefotaxime, and levofloxacin, whereas resistance was observed for tetracycline, erythromycin, and clindamycin. The percentage of overall tetracycline resistance among GAS isolates in this study was 13.6%, which is comparable to what has been reported in other studies (7, 12, 15, 18). However, tetracycline resistance was significantly higher during the early surveillance years (23.6%; 2004 to 2008) than in the later surveillance years (3.7%; 2009 to 2013) (P < 0.001). This decline was the result of a decrease in the number of emm87 isolates in the later surveillance years. The proportion of GAS isolates that were resistant to erythromycin (5.8%) was comparable to what has been reported in other studies (15, 17, 42, 46). The majority (65%) of erythromycin-resistant isolates belonged to emm types 58 and 92. Other studies have found erythromycin resistance associated with emm types 4, 11, and 28 (15, 16, 18, 21, 42). Resistance to both tetracycline and erythromycin was associated with emm types 58 and 94, of which emm58 was more common (78%).
In conclusion, this is the first report on the epidemiology of invasive GAS disease and emm type distribution in Alaska. The overall annual incidence of GAS in Alaska is slightly higher than reported in other populations, which is most likely driven by higher rates of disease among the AN population. As reported elsewhere, we found significant variation in emm type distribution, which warrants continued surveillance to assess the potential utility of proposed GAS vaccines.
ACKNOWLEDGMENTS
We thank the clinicians and microbiology laboratory personnel of the hospitals participating in statewide surveillance for invasive group A streptococcal disease in Alaska. We also thank the entire staff at the Arctic Investigations Program for their contributions to this study.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Cunningham MW. 2000. Pathogenesis of group A streptococci. Clin Microbiol Rev 13:470–491. doi: 10.1128/CMR.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect Dis 5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 3.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. 2009. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet 9:611–616. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 4.Carapetis JR, Robins-Browne R, Martin D, Shelby-James T, Hogg G. 1995. Increasing severity of invasive group A streptococcal disease in Australia: clinical and molecular epidemiological features and identification of a new virulent M-nontypeable clone. Clin Infect Dis 21:1220–1227. doi: 10.1093/clinids/21.5.1220. [DOI] [PubMed] [Google Scholar]
- 5.Colman G, Tanna A, Efstratiou A, Gaworzewska ET. 1993. The serotypes of Streptococcus pyogenes present in Britain during 1980-1990 and their association with disease. J Med Microbiol 39:165–178. doi: 10.1099/00222615-39-3-165. [DOI] [PubMed] [Google Scholar]
- 6.Holm SE, Norrby A, Bergholm AM, Norgren M. 1992. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988-1989. J Infect Dis 166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Martin J, Murchan S, O'Flanagan D, Fitzpatrick F. 2011. Invasive group A streptococcal disease in Ireland, 2004 to 2010. Eurosurveillance 16:1–7. [PubMed] [Google Scholar]
- 8.O'Brien KL, Beall B, Barrett NL, Cieslak PR, Reingold A, Farley MM, Danila R, Zell ER, Facklam R, Schwartz B, Schuchat A, Active Bacterial Core Surveillance/Emerging Infections Program Network. 2002. Epidemiology of invasive group A Streptococcus disease in the United States, 1995-1999. Clin Infect Dis 35:268–276. doi: 10.1086/341409. [DOI] [PubMed] [Google Scholar]
- 9.O'Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, Farley MM, Barrett NL, Spina NL, Beall B, Harrison LH, Reingold A, Van Beneden C, Active Bacterial Core Surveillance Team. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis 45:853–862. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 10.Athey TBT, Teatero S, Li A, Marchand-Austin A, Beall B, Fittipaldi N. 2014. Deriving group A streptococcus typing information from short-read whole-genome sequencing data. J Clin Microbiol 52:1871–1876. doi: 10.1128/JCM.00029-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beall B, Facklam R, Thompson T. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol 34:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creti R, Imperi M, Baldassarri L, Pataracchia M, Recchia S, Alfarone G, Orefici G. 2007. emm types, virulence factors, and antibiotic resistance of invasive Streptococcus pyogenes isolates from Italy: what has changed in 11 years? J Clin Microbiol 45:2249–2256. doi: 10.1128/JCM.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darenberg J, Luca-Harari B, Jasir A, Sandgren A, Pettersson H, Schalén C, Norgren M, Romanus V, Norrby-Teglund A, Normark BH. 2007. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis 45:450–458. doi: 10.1086/519936. [DOI] [PubMed] [Google Scholar]
- 14.Ekelund K, Darenberg J, Norrby-Teglund A, Hoffmann S, Bang D, Skinhøj P, Konradsen H. 2005. Variations in emm type among group A streptococcal isolates causing invasive and noninvasive infections in a nationwide study. J Clin Microbiol 43:3101–3109. doi: 10.1128/JCM.43.7.3101-3109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imöhl M, Reinert RR, Ocklenburg C, van der Linden M. 2010. Epidemiology of invasive Streptococcus pyogenes disease in Germany during 2003-2007. FEMS Immunol Med Microbiol 58:389–396. doi: 10.1111/j.1574-695X.2010.00652.x. [DOI] [PubMed] [Google Scholar]
- 16.Krucsó B, Gacs M, Libisch B, Hunyadi ZV, Molnár K, Füzi M, Pászti J. 2007. Molecular characterization of invasive Streptococcus pyogenes isolates from Hungary obtained in 2004 and 2005. Eur J Clin Microbiol Infect Dis 26:807–811. doi: 10.1007/s10096-007-0359-4. [DOI] [PubMed] [Google Scholar]
- 17.Meisal R, Høiby EA, Aaberge IS, Caugant DA. 2008. Sequence type and emm type diversity in Streptococcus pyogenes isolates causing invasive disease in Norway between 1988 and 2003. J Clin Microbiol 46:2102–2105. doi: 10.1128/JCM.00363-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montes M, Ardanuy C, Tamayo E, Domènech A, Liñares J, Pérez-Trallero E. 2011. Epidemiology and molecular analysis of Streptococcus pyogenes isolates causing invasive disease in Spain (1998-2009): comparison with non-invasive isolates. Eur J Clin Microbiol Infect Dis 30:1295–1302. doi: 10.1007/s10096-011-1226-x. [DOI] [PubMed] [Google Scholar]
- 19.O'Grady KF, Kelpie L, Andrews RM, Curtis N, Nolan TM, Selvaraj G, Passmore JW, Oppedisano F, Carnie JA, Carapetis JR. 2007. The epidemiology of invasive group A streptococcal disease in Victoria, Australia. Med J Australia 186:565–569. [DOI] [PubMed] [Google Scholar]
- 20.Siljander T, Lyytikäinen O, Vähäkuopus S, Snellman M, Jalava J, Vuopio J. 2010. Epidemiology, outcome and emm types of invasive group A streptococcal infections in Finland. Eur J Clin Microbiol Infect Dis 29:1229–1235. doi: 10.1007/s10096-010-0989-9. [DOI] [PubMed] [Google Scholar]
- 21.Stathi A, Papaparaskevas J, Zachariadou L, Pangalis A, Legakis NJ, Tseleni-Kotsovili A, Tassios PT, the Hellenic Strep-EURO Study Group. 2008. Prevalence of emm types 1 and 12 from invasive Streptococcus pyogenes disease in Greece—results of enhanced surveillance. Clin Microbiol Infect 14:808–812. doi: 10.1111/j.1469-0691.2008.02032.x. [DOI] [PubMed] [Google Scholar]
- 22.Szczypa K, Sadowy E, Isdebski R, Strakove L, Hryniewicz W. 2006. Group A streptococci from invasive-disease episodes in Poland are remarkably divergent at the molecular level. J Clin Microbiol 44:3975–3979. doi: 10.1128/JCM.01163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baird RW, Bronze MS, Kraus W, Hill HR, Veasey LG, Dale JB. 1991. Epitopes of group A streptococcal M protein shared with antigens of articular cartilage and synovium. J Immunol 146:132–137. [PubMed] [Google Scholar]
- 24.Bronze MS, Dale JB. 1993. Epitopes of streptococcal M proteins that evoke antibodies that cross-react with human brain. J Immunol 151:2820–2828. [PubMed] [Google Scholar]
- 25.Dale JB, Penfound T, Chiang EY, Long V, Shulman ST, Beall B. 2005. Multivalent group A streptococcal vaccine elicits bactericidal antibodies against variant M subtypes. Clin Diagn Lab Immunol 12:833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu MC, Walls MA, Stroop SD, Reddish MA, Beall B, Dale JB. 2002. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect Immun 70:2171–2177. doi: 10.1128/IAI.70.4.2171-2177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotloff KL, Corretti M, Palmer K, Campbell JD, Reddish MA, Hu MC, Wasserman SS, Dale JB. 2004. Safety and immunogenicity of a recombinant multivalent group A streptococcal vaccine in healthy adults: phase 1 trial. JAMA 292:709–715. doi: 10.1001/jama.292.6.709. [DOI] [PubMed] [Google Scholar]
- 28.Olive C, Hsien K, Horváth A, Clair T, Yarwood P, Toth I, Good MF. 2005. Protection against group A streptococcal infection by vaccination with self-adjuvanting lipid core M protein peptides. Vaccine 23:2298–2303. doi: 10.1016/j.vaccine.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 29.McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, Baxendale DM, Reddish MA, Hu MC, Stroop SD, Linden J, Fries LF, Vink PE, Dale JB. 2005. Safety and immunogenicity of 26-valent group A Streptococcus vaccine in health adult volunteers. Clin Infect Dis 41:1114–1122. doi: 10.1086/444458. [DOI] [PubMed] [Google Scholar]
- 30.Dale JB, Penfound TA, Chiang EY, William WJ. 2011. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 29:8175–8178. doi: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dale JB, Penfound TA, Tamboura B, Sow SO, Nataro JP, Tapia M, Kotloff KL. 2013. Potential coverage of a multivalent M protein-based group A streptococcal vaccine. Vaccine 31:1576–1581. doi: 10.1016/j.vaccine.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. 2011. Performance standards for antimicrobial susceptibility testing. Twenty-first informational supplement M100-S21, vol. 31. Clinical and Laboratory Stantards Institute, Wayne, PA. [Google Scholar]
- 33.State of Alaska Department of Labor and Workforce Development. 2014. http://www.labor.alaska.gov/research/pop/popest.htm Accessed 11 February 2015.
- 34.Simpson EH. 1949. Mesurement of diversity. Nature 163:688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- 35.Magurran AE. 2004. Measuring biological diversity. Blackwell Publishing, Oxford, United Kingdom. [Google Scholar]
- 36.Vlaminckx BJM, van Pelt W, Schouls LM, van Silfhout A, Mascini EM, Elzenaar CP, Fernandes T, Bosman A, Schellekens JFP. 2005. Long-term surveillance of invasive group A streptococcal disease in The Netherlands, 1994-2003. Clin Microbiol Infect 11:226–231. doi: 10.1111/j.1469-0691.2004.01068.x. [DOI] [PubMed] [Google Scholar]
- 37.Stockmann C, Ampofo K, Hersh AL, Blaschke AJ, Kendall BA, Korgenski K, Daly J, Hill HR, Byington CL, Pavia AT. 2012. Evolving epidemiologic characteristics of invasive group a streptococcal disease in Utah, 2002-2010. Clin Infect Dis 55:479–487. doi: 10.1093/cid/cis422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norton R, Smith RV, Wood N, Siegbrecht E, Ross A, Ketheesan N. 2004. Invasive group A streptococcal disease in North Queensland (1996-2001). Indian J Med Res 119(Suppl):148–151. [PubMed] [Google Scholar]
- 39.Safar A, Lennon D, Stewart J, Trenholme A, Drinkovic D, Peat B, Taylor S, Read K, Roberts S, Voss L. 2011. Invasive group A streptococcal infection and vaccine implications, Auckland, New Zealand. Emerg Infect Dis 17:983–989. doi: 10.3201/eid/1706.100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steer AC, Jenney A, Kado J, Good MF, Batzloff M, Waqatakirewz L, Mullholland EK, Carapetis JR. 2009. Prospective surveillance of invasive group A streptococcal disease in Fiji, 2005-2007. Emerg Infect Dis 15:216–222. doi: 10.3201/eid15/2.080558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamagni TL, Darenberg J, Luca-Harari B, Siljander T, Efstratiou A, Henriques-Normark B, Vuopio-Varkila J, Bouvet A, Creti R, Ekelund K, Koliou M, Reinert RR, Stathi A, Strakova L, Ungureanu V, Schalén C, the Strep-EURO Study Group, Jasir A. 2008. Epidemiology of severe Streptcococcus pyogenes disease in Europe. J Clin Microbiol 46:2359–2367. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luca-Harari B, Ekelund K, van der Linden M, Staum-Kaltoft M, Hammerum A, Jasir A. 2008. Clinical and epidemiological aspects of invasive Streptococcus pyogenes infections in Denmark during 2003 and 2004. J Clin Microbiol 46:79–86. doi: 10.1128/JCM.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ato M, Ikebe T, Kawabata H, Takemori T, Watanabe H. 2008. Incompetence of neutrophils to invasive group A streptococcus is attributed to induction of plural virulence factors by dysfunction of a regulator. PLoS One 3:e3455. doi: 10.1371/journal.pone.0003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikebe T, Endoh M, Watanabe H. 2005. Increased expression of the ska gene in emm49-genotyped Streptococcus pyogenes strains isolates from patients with severe invasive streptococcal infections. Jpn J Infect Dis 58:272–275. [PubMed] [Google Scholar]
- 45.Sanderson-Smith M, De Oliveira DMP, Guglielmini J, McMillan DJ, Vu T, Holien JK, Henningham A, Steer AC, Bessen DE, Dale JB, Curtis N, Beall BW, Walker MJ, Parker MW, Carapetis JR, Van Melderen L, Sriprakash KS, Smeesters PR, M Protein Study Group. 2014. A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J Infect Dis 210:1325–1338. doi: 10.1093/infdis/jiu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter SS, Heilmann KP, Beekmann SE, Miller NJ, Miller AL, Rice CL, Doern CD, Reid SD, Doern GV. 2005. Macrolide-resistant Streptococcus pyogenes in the United States, 2002-2003. Clin Infect Dis 41:599–608. doi: 10.1086/432473. [DOI] [PubMed] [Google Scholar]