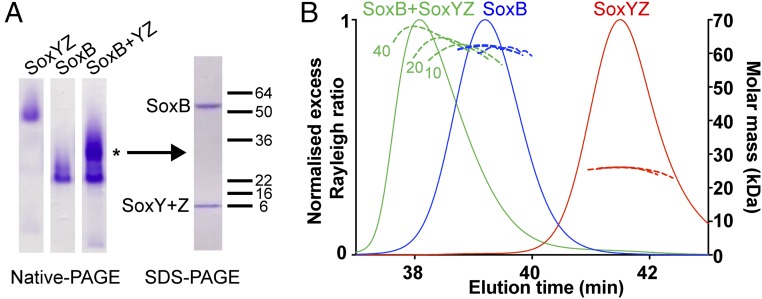
Fig. 3.
SoxYZ forms a weak complex with SoxB. (A) Comparative native PAGE analysis of SoxYC151SZ, SoxB, or a mixture of the two proteins (Left). Each sample analyzed contained 10 μM (per component) protein. Native PAGE used the Laemmli buffer system (20) and a 7% polyacrylamide gel. A major band present only in the mixed sample is indicated with *. The polypeptide composition of this band was determined by excising the band, dehydrating the gel slice in acetonitrile followed by boiling in SDS-containing buffer and analysis on an SDS/PAGE gel (Right). Note that T. thermophilus SoxY and SoxZ have identical electrophoretic mobilities in SDS/PAGE. (B) SEC-MALLS analysis of SoxYC151SZ, SoxB, or mixtures of the two proteins. Measured average molar masses are shown for loading concentrations (per protein component) of 10, 20, and 40 μM (dashed lines, with the loading concentrations indicated for the SoxB/SoxYC151SZ mixture). The normalized excess Rayleigh ratio is shown for the 40 μM concentration samples only (solid lines).