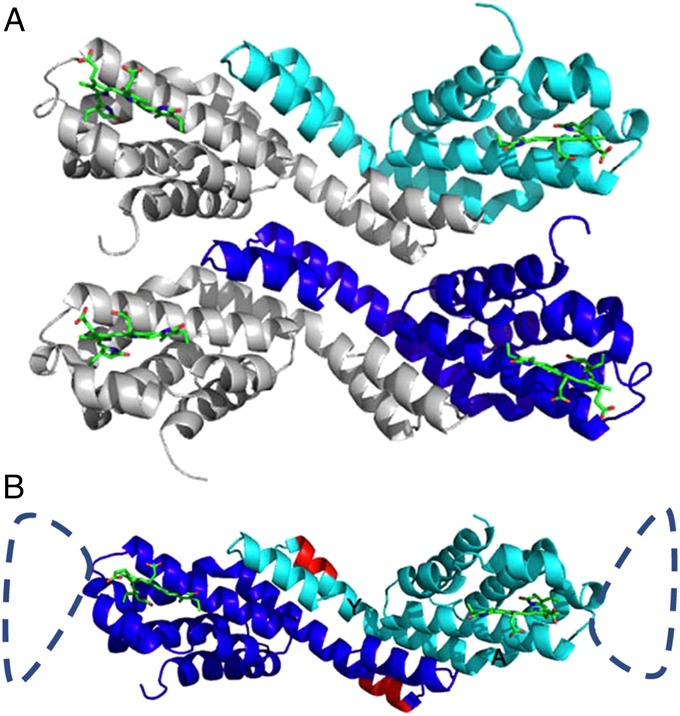
Fig. 2.
(A) Structure of ApcEΔ bearing the PCB chromophore and arrangement of functional “homo”-dimers (chains A and B) in the crystal. Chain A is shown in cyan, and chain B is shown in blue. In the asymmetrical unit, the two chains are arranged in parallel. (B) Asymmetrical unit of ApcEΔ-Se containing a functional homodimer of chains A and B. The homodimers are similar to phycobiliprotein αβ-heterodimers; the position of the deleted amino acids 77–153 is indicated by the dashed loop. The PCB chromophores (green) in both monomers are shown as stick-and-ball presentations. Amino acids 38–39 and 41–42 (red), which have been deleted in the construct ApcEΔΔ, are located in helix Y (labeling shown in B). The angle between helices Y and A is relevant for the distance between two chromophores in the homodimer, and the positioning of the loop.