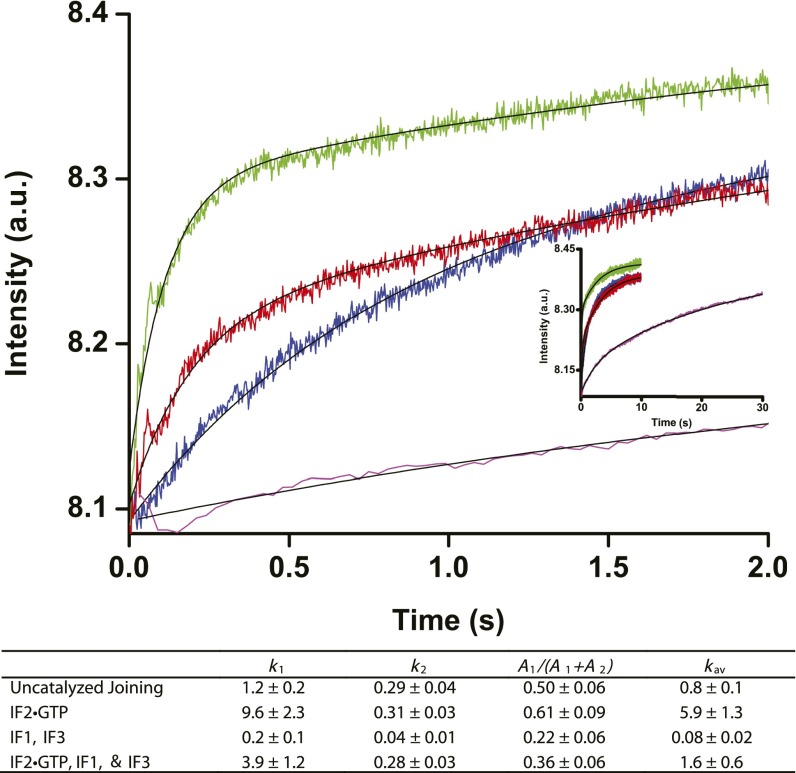
Fig. S2.
Ensemble kinetics of 50S ribosomal subunit binding to 30S ribosomal subunit complexes. 50S ribosomal subunits (100 nM after mixing) were mixed using a stopped flow apparatus with 30S ribosomal subunits (50 nM after mixing) preincubated with mRNA and initiator fMet-tRNAfMet (blue trace); 30S ICs assembled in the presence of mRNA, initiator fMet-tRNAfMet and IF2•GTP (green trace); 30S ICs assembled in the presence of mRNA, initiator fMet-tRNAfMet, IF1, IF3, and IF2•GTP (red trace); 30S IC assembled in the presence of mRNA, initiator fMet-tRNAfMet, IF1 and IF3 (magenta trace). Subunit joining was monitored by light scattering as a function of time. (Inset) Kinetic traces at longer time scale set out to 30 s. Consistent with previous reports (28, 49), the kinetics of subunit association were biphasic and are best fitted to the sum of two exponentials, corresponding to the apparent rate constants k1 (s−1) and k2 (s−1). Double-exponential fits are black lines. The rate constants, k1 and k2, and their respective amplitudes, A1 and A2, were calculated from double-exponential fits. The contribution of the fast phase to the total amplitude of the increase in light scattering was calculated as A1/(A1 + A2). The weighted average of the overall rate, kav (s−1), was calculated as kav = (k1A1 + k2A2)/(A1 + A2). The kav of 50S subunit association to 30S ICs containing all IFs, fMet-tRNAfMet, and GTP calculated in this study was nearly identical to the average overall rate of 50S subunit association to 30S ICs observed previously in other studies under similar conditions (28, 49).