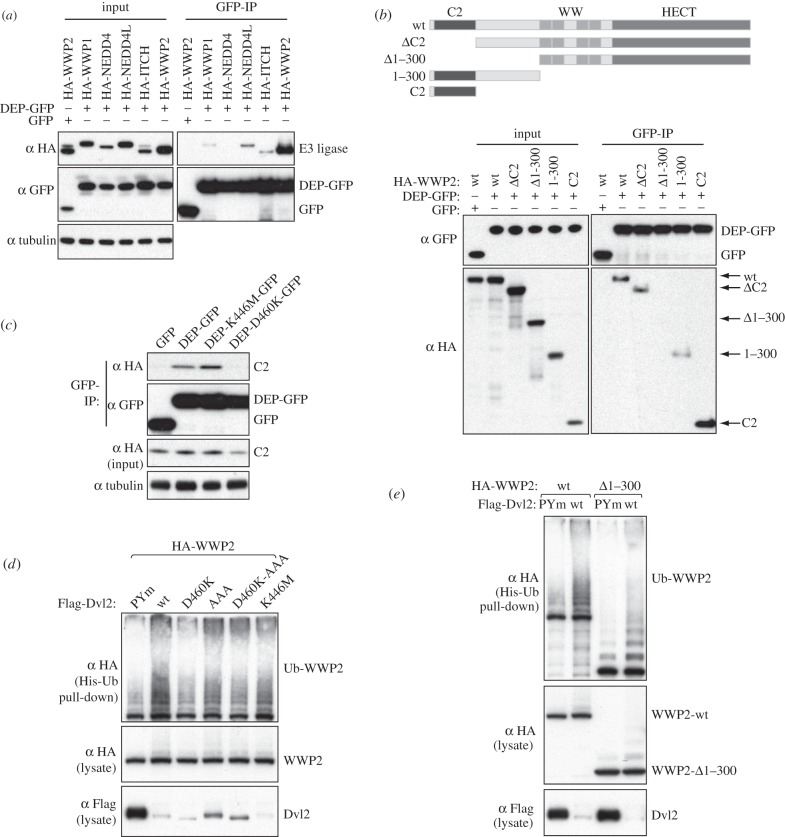
Figure 4.
Functional interaction between the Dvl2 DEP domain and the WWP2 C2 domain. (a–c) Western blots of coIPs from HEK293T cells co-transfected with GFP-tagged DEP domain of Dvl2 and (a) different HA-tagged NEDD4 family ligases, (b) various truncations of HA-WWP2 and (c) HA-tagged C2 domain of WWP2, as indicated above panels. Dvl2-DEP exhibits a strong preference for WWP2 (a), and its binding to the WWP2 C2 domain (b) is blocked by a single point mutation (D460K) in a solvent-exposed surface residue within the second a helix of the DEP domain (c). (d) Western blot of His pull-downs from HEK293T cells co-transfected with His-Ub, wt or mutant Flag-Dvl2 and wt HA-WWP2, as indicated above panels. Note that D460K activates less well than wt, revealing a contribution of the Dvl2 DEP domain to the disinhibition of WWP2. (e) His pull-downs showing that wt and N-terminally truncated WWP2 are efficiently disinhibited by wt but not PPxY-mutant Dvl2.