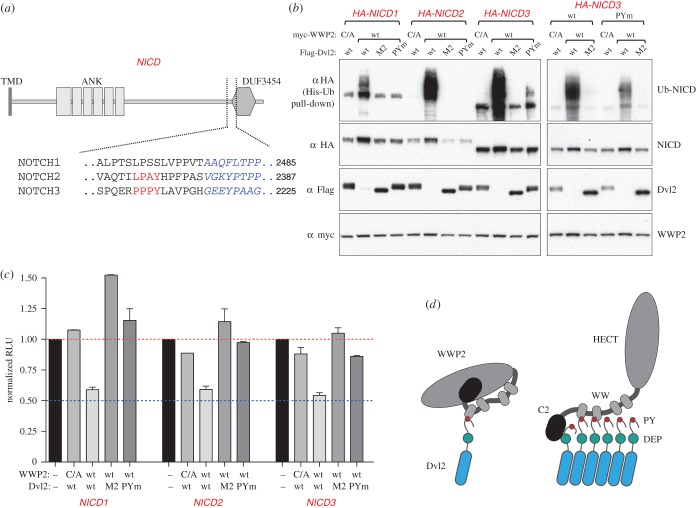
Figure 8.
Ubiquitylation of Notch by Dvl2-activated WWP2 attenuates its activity. (a) Cartoon of the intracellular domain of Notch (NICD) and its domains, with PPxY elements and flanking sequences in human Notch2 and Notch3 underneath (no PPxY-like motifs are detectable in Notch1); TMD, transmembrane domain; ANK, ankyrin repeats; DUF3454, conserved domain of unknown function. (b) Western blot of His pull-downs from HEK293T cells co-transfected with His-Ub, wt HA-NICD (from Notch1, Notch2 and Notch3) or PPxY-mutant NICD (from Notch3), wt or catalytically inactive HA-WWP2 and wt, PPxY mutant (PYm) or polymerization-deficient (M2) Flag-Dvl2, as indicated above panels; note that efficient ubiquitylation of NICD conferred by WWP2 depends on its disinhibition by polymerization-competent Dvl2, on the PPxY element of Dvl2 and also, to some extent, on the PPxY element of NICD3. Note also that the low level of ubiquitylation of the PPxY-less NICD1 is comparable to the reduced level of NICD3 ubiquitylation in the absence of its PPxY, both of which are strictly dependent on polymerized Dvl2 (which may interact with NICD directly, see text). (c) Reporter assays in HEK293T cells co-transfected with a miminal Notch reporter (4xCBS-Luc), HA-NICD (from Notch1, Notch2 and Notch3), wt or catalytically inactive HA-WWP2 and wt or mutant Flag-Dvl2, revealing Dvl2-dependent reduction of the transcriptional activity of NICD. RLU, relative luciferase units (normalized to NICD-dependent activity in the absence of WWP2 and Dvl2); error bars, mean±s.d. (d) Disinhibition of WWP2 by polymerized Dishevelled: a model. Left, unpolymerized Dishevelled undergoes a single PPxY–WW interaction with WWP2, which is compatible with simultaneous autoinhibitory intramolecular C2–HECT and WW–HECT interactions. Right, DIX-mediated polymerization (triggered by Wnt stimulation, or high expression levels of Dishevelled) generates a high local concentration of Dishevelled, allowing WWP2 to undergo multiple simultaneous WW–PPxY and DEP–C2 interactions with adjacent Dishevelled molecules, which compete with the autoinhibitory intramolecular interactions of WWP2, thus exposing its HECT domain and allowing it to interact with E2 ligase and substrates.