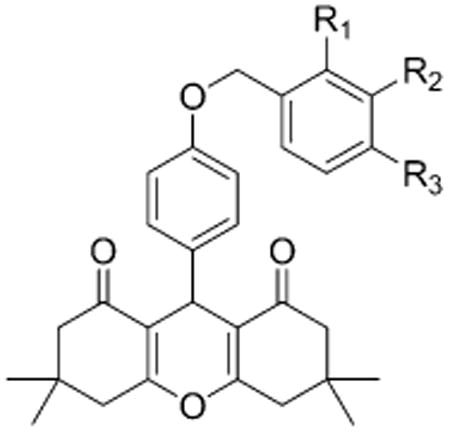
Table 1. Structure-Activity Relationship of the δ-PAM Chemotype in PathHunter CHO-OPRD1 and CHO-OPRM1 Cells in a β-Arrestin Recruitment Assaya.
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| EC50 (μM) (% Ymax) | ||||||
|
|
||||||
| compd | R1 | R2 | R3 | δ | μ | selectivity μ/δ |
| 1 | H | H | H | 0.2 (126) | 6 (130) | 30 |
| 2 (BMS-986187) | CH3 | H | H | 0.03 (124) | 3 (121) | 100 |
| 3 | H | CH3 | H | 0.2 (136) | 3 (120) | 15 |
| 4 | H | H | CH3 | 0.3 (92) | 4 (65) | 13 |
| 5 | F | H | H | 0.1 (120) | 5 (107) | 50 |
| 6 | H | F | H | 1 (136) | 7 (182) | 7 |
| 7 | H | H | F | 0.1 (95) | 2 (72) | 20 |
| 8 | Cl | H | H | 0.3 (68) | >10 (>100) | >33 |
| 9 | Cl | Cl | H | 0.1 (116) | >10 (>87) | >100 |
| 10 (BMS-986188) | Br | H | H | 0.05 (58) | >10 (>20) | >200 |
| 11 | OCHF2 | H | H | 0.2 (109) | 3 (80) | 15 |
| 12 | OCF3 | H | H | 0.3 (114) | 2 (111) | 7 |
| 13 | S02CH3 | H | H | 0.9 (102) | 5 (42) | 6 |
| 14 | CH2OH | H | H | 1 (92) | 10 (112) | 10 |
| 15 | CF3 | H | H | >10 (>40) | >10 (>20) | |
No activity was observed in agonist mode (in the absence of orthosteric agonist (data not shown)). In PAM mode (in the presence of an EC20 of leu-enkephalin for OPRD1 cells or an EC20 of endomorphin I for OPRM1 cells), robust responses were observed. The mean EC50 values, Ymax values, and potency ratio of δ receptor activity/μ receptor activity in PAM mode are reported in the table (n = 3).
