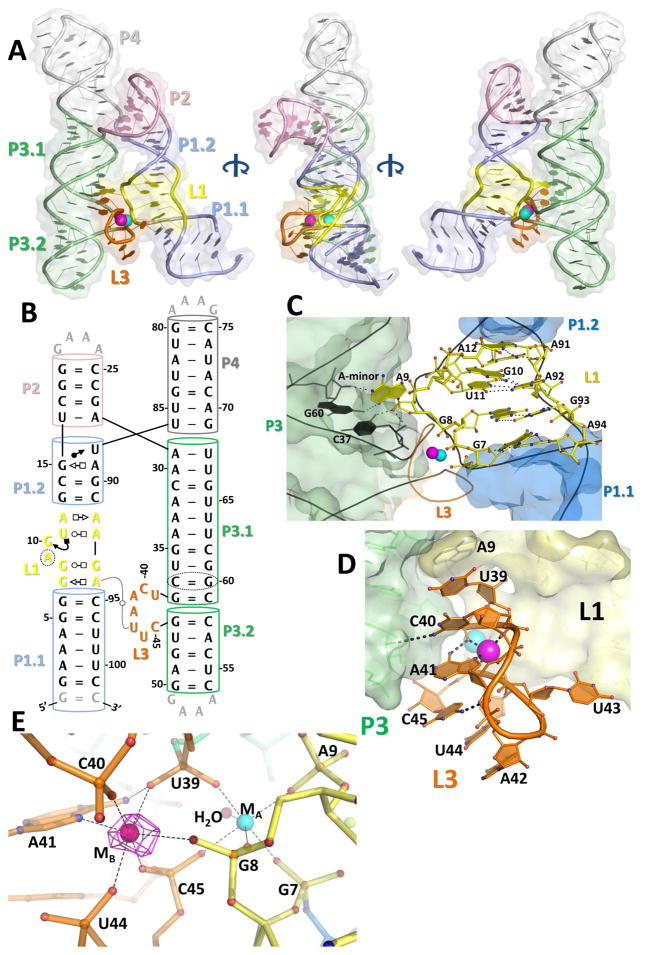
Figure 2. The crystal structure of the L. lactis yybP-ykoY aptamer domain bound to Mn2+.
A) Overall 3-D structure shows two series of coaxially-stacked helices forming an overall hairpin shape, with the highly conserved L1 (yellow) and L3 (orange) docking together and binding two metal ions (cyan and bright pink). B) Updated secondary structure reflecting the 3-D structure. Numbering is by the wild-type sequence (not crystal construct). C) The L1 region contains a series of non-Watson-Crick interactions helping to arrange the metal binding site. Notably, G7 and G8 phosphates are pointed toward the metal binding site. Additionally, A9 is flipped-out to make an A-minor interaction with C37-G60 and base stack with top of L3. D) The L3 loop contains the other half of the binding site. Extensive base stacking, and hydrogen bonds from C40 to 2′-OH of G46 and N6 of A41 to phosphoryl oxygen of U39, help arrange A41 and backbone phosphate to contact the metals. E) The metal coordination scheme. MA (cyan) is coordinated octahedrally by five backbone phosphates and a water. The MB site (bright pink) contains five backbone phosphates and the N7 of A41. N6 of A41 also makes a H-bond to the phosphate of U39. The anomalous difference map collected at the Mn K-edge (magenta, contoured to 12 σ) with 100 μM Mn2+ confirms that MB is Mn2+-specific.