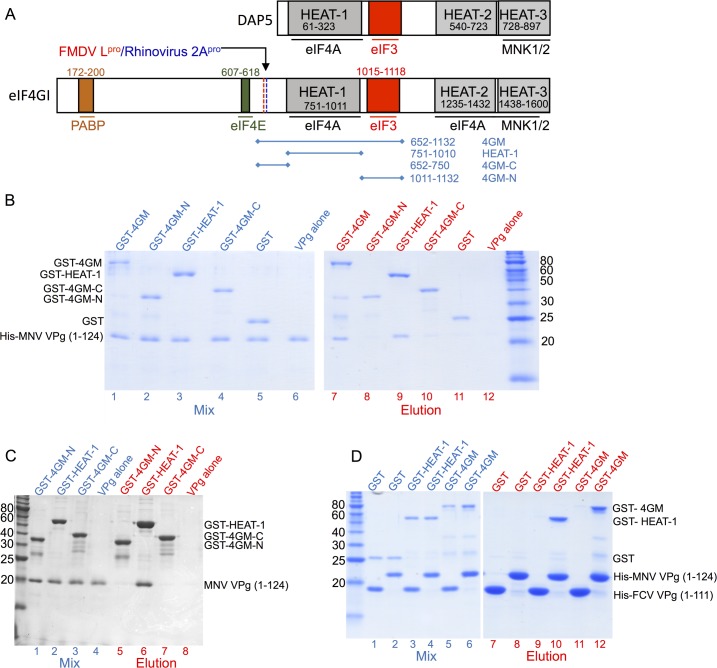
Fig 1. MNV VPg interacts with eIF4GI via its HEAT-1 domain.
(A) Schematic representation of eIF4GI (NCBI accession AAM69365.1), one of two eIF4G paralogues expressed in humans, and the paralog DAP5 (NCBI accession NP_001036024.3). Positions of domains that interact with other proteins of the translation initiation machinery are indicated, as are the cleavage sites of FMDV L protease and Rhinovirus 2A protease. The principal eIF4G fragments that were sub-cloned for use in this study are also indicated. (B) SDS PAGE analysis of glutathione affinity pull-down assays that were performed to map the locus of MNV VPg binding. GST-fusions of various eIF4GI fragments (shown in panel A) were used as bait and His-tagged-MNV VPg(1–124) as prey. Left panel: protein mixtures applied to the glutathione-sepharose 4B beads (lanes 1–6). Right panel: proteins eluted with 10 mM glutathione (lanes 7–12). (C) SDS PAGE analysis of cobalt affinity pull-down assays to confirm that binding of MNV VPg occurs primarily through the eIF4G HEAT-1 domain. GST-fusions of various eIF4GI fragments with C-terminal His-tags were used as bait and untagged MNV VPg as prey. Lanes 1–4: protein mixtures applied to the cobalt resin; lanes 5–8: proteins eluted with 250 mM imidazole. (D) SDS PAGE analysis of cobalt affinity pull-down assays performed to confirm the eIF4G HEAT-1 domain as the locus of MNV VPg binding. His-tagged-MNV VPg(1–124) or His-tagged-FCV VPg(1–111) were used as bait proteins and GST-fusions of various eIF4GI fragments as prey. Left panel: protein mixtures applied to the cobalt resin (Lanes 1–6). Right panel: proteins eluted with 250 mM imidazole (lanes 7–12).