Abstract
Purpose
Loss of CD46 has recently been implicated in choroidal neovascularization in mice. Herein we investigated the effect of nitrite modification of the extracellular matrix (ECM) as an in vitro model of “aging” and its effect on CD46 expression and vascular endothelial growth factor (VEGF) release in cocultured human retinal pigment epithelium (RPE).
Methods
ARPE-19 cells were plated onto RPE-derived ECM conditions (untreated; nitrite modified; nitrite modified followed by washing with Triton X-100; or nitrite modified followed by washing with Triton X-100 and coated with extracellular matrix ligands). Cells were cultured for 7 days and CD46 expression was analyzed by immunohistochemistry and Western blot. Additionally, CD46 short interfering RNA (siRNA) was transfected into ARPE-19 cells, and VEGF levels were determined by ELISA. Finally, in the same ECM conditions, ARPE-19 cells were challenged with normal human serum and VEGF levels determined by ELISA.
Results
CD46 is expressed on the basolateral surface of ARPE-19 cells on RPE-derived ECM. Nitrite modification of ECM reduced the expression of CD46 on ARPE-19 cells by 0.5-fold (P = 0.003) and increased VEGF release in ARPE-19 cells by 1.7-fold (P < 0.001). CD46 knockdown also increased release of VEGF on the apical and basal sides of ARPE-19 cells in culture by 1.3- (P = 0.012) and 1.2-fold (P = 0.017), respectively.
Conclusions
Nitrite modification of the ECM decreased CD46 expression and increased the release of VEGF from ARPE-19 cells. Changes in CD46 expression may lead to changes in VEGF and play a pathologic role in the development of age-related macular degeneration.
Keywords: age-related macular degeneration, retinal pigment epithelium, CD46, vascular endothelial growth factor, Bruch's membrane
We determined the effect of nitrite modification of the ECM, which is an in vitro model of “aging,” on CD46 expression and release of VEGF by human RPE cells. We also demonstrated a role for CD46 in the regulation of VEGF, by knocking down CD46 in RPE cells and measuring changes in VEGF release.
Age-related macular degeneration (AMD) is a leading cause of blindness in the United States and Western Europe.1 Among patients older than 70 years, 30% to 50% will have some evidence of this disease, and severe loss of vision is present in 2%. Loss of vision in the setting of advanced AMD results from either choroidal neovascularization (CNV; “exudative” or “wet” AMD) or geographic atrophy (GA). Geographic atrophy involves a well-defined area of loss of retinal pigment epithelium (RPE), underlying choriocapillaris, and thinning of the overlying neurosensory retina.2 The precise mechanisms underlying both AMD phenotypes are not well understood.
Age-related damage to RPE is associated with changes to Bruch's membrane (BM), which serves as a substrate for RPE attachment.3 Age-related changes in BM include diffuse thickening, accumulation of drusen, basal laminar deposits, basal linear deposits,4,5 collagen cross-linking in the inner and outer collagen layer, calcification and fragmentation of the elastin layer,6 and lipidization.6–8 These changes precede RPE cellular changes and dysfunction by 1 to 2 decades in AMD patients.4,9 Age-related changes in human BM induce multiple deleterious effects on RPE function, including cell adhesion, proliferation, differentiation, migration, and the ability to phagocytize outer segments.3,4,7,9,10 Chronic inflammation, which is strongly associated with AMD, contributes to these structural changes in BM and can have additional deleterious effects on RPE fate, thus leading to RPE loss and eventual atrophy.3,11–16
The production of nitric oxide, a cross-linking agent, has been implicated in chronic inflammation and the production of its byproducts such as nitrite, thus subjecting these areas to increased nitrosative stress. Another contributor to chronic nitric oxide production and subsequent nitrite exposure is cigarette smoking, a risk factor strongly associated with AMD.17,18 These changes contribute to the cumulative damage to the BM and result in the age-related collagen cross linking, a decline in collagen solubility, and subsequent membrane damage.3,16
Model systems that mimic the effects of BM aging have been used with success to determine the contribution of extracellular matrix (ECM) damage on the cellular function and pathology of the overlying RPE.3,19 Previously, we have demonstrated that nonenzymatic nitration of basement membrane proteins can serve as a relevant model of BM aging.3 Nonenzymatic nitration of ECM proteins induces RPE dysfunction in vitro, thus making this model pertinent to the age-related changes seen in the basement membrane and adjacent cells in macular degeneration.3 Additionally, the deleterious effects of basement membrane aging on RPE function can be reversed by cleaning the previously nitrite-modified ECM surface combined with additional ECM protein coating.3,20
Activation of the complement system has been implicated in the pathogenesis of AMD and is thought to contribute to the pathologic changes that develop in the macula in this disease.11–13,21–23 Activated complement fragments contribute to the induction of vascular endothelial growth factor (VEGF) in RPE cells and in animal models of AMD,24,25 and dysregulation of VEGF contributes to the abnormal blood vessel growth seen in CNV.26 Complement regulatory proteins help to control the complement cascade and have been linked to the regulation of CNV in animal models of laser-induced CNV.27–29 The RPE expresses several complement molecules including CD46 or membrane cofactor protein (MCP), which is a complement regulatory protein located at the same subband on the same chromosome (1q32) as complement factor H within a complex of immunoregulatory genes.30,31 CD46 is expressed on the basal surface of the RPE, and thus occupies an important anatomic position between the pigment epithelium and the choriocapillaris external to the outer blood–retina barrier.32,33 CD46 is a key regulator of the alternative complement pathway that acts as a surface-bound, immobilized inhibitor of complement on the basal surface of the RPE.34–38 Interestingly, Vogt et al.33 have found that CD46 is the only membrane-bound regulator of complement activation detected by immunohistochemistry on the human RPE basolateral surface.
This study determined the relationship between age-related changes in the basement membrane, complement activation, and the release of VEGF by human RPE cells. Specifically, we determined the effect of nitrite modification of the ECM, which is an in vitro model of ECM “aging,” on the expression of CD46 by human RPE cells. Additionally, we demonstrated a role for CD46 in the regulation of VEGF, by knocking down CD46 in human RPE cells and measuring the subsequent changes in VEGF release.
Methods
Cell Culture
Immortalized human RPE (ARPE-19) cells obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured and propagated in Dulbecco's modified Eagle's medium (DMEM; Invitrogen-Gibco, Life Technologies, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS), 100 IU/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL gentamicin, and 2.5 μg/mL amphotericin B (Invitrogen-Gibco, Life Technologies). The cells were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C, and the culture medium was replaced every other day. These ARPE-19 cells were used for downstream experiments where they were either plated onto ECM, or were transfected with short interfering RNA (siRNA) (described below).
Preparation of ECM
To prepare the RPE ECM nitrite-treated plates, we used a method previously described.19,20 Briefly, ARPE-19 cells were grown on 24-well Transwell permeable supports (Corning, Tewksbury, MA, USA) in 12-well plates for 6 to 8 weeks to allow for ECM formation. ARPE-19 cells were removed by the addition of 20 mM ammonium hydroxide buffer for 20 minutes, and the ECM was washed three times with phosphate-buffered saline (PBS). Phosphate-buffered saline was removed from the RPE-derived ECM (RPE-ECM), which was then allowed to dry. The RPE-derived ECM plates were either used immediately after drying, or stored at −20°C for future use. RPE-derived ECM was processed further to create four experimental plating surfaces (untreated, nitrite modified, nitrite modified followed by washing, or nitrite modified followed by washing and coating with a mixture of ECM ligands).
Nitrite Modification
Nitrite-modified ECM was prepared by adding 100 mM sodium nitrite to the ECM and incubating at 37°C for 7 days. Plates were then washed at least four times with PBS and further incubated with PBS for 4 hours. Following this, the plates were washed at least two additional times to completely remove the nitrite.
Extracellular Matrix Detergent Cleaning.
Triplicate wells were treated with 0.1% Triton X-100/0.1% sodium citrate solution for 20 minutes at 4°C. After cleaning, the ECM was washed three times with PBS for 5 minutes and prepared for plating of ARPE-19 cells.
Addition of ECM Proteins.
All ECM in this treatment condition was detergent cleaned as described above. Following this, triplicate wells were incubated with an ECM–protein mixture containing laminin (330 μg/mL; Sigma-Aldrich Corp., St. Louis, MO, USA), fibronectin (250 μg/mL, Sigma-Aldrich Corp.), and vitronectin (33 μg/mL; Corning Incorporated Life Sciences, Tewksbury, MA, USA) at 37°C for 30 minutes. The ECM was then washed three times with PBS for 5 minutes and prepared for plating of ARPE-19 cells.
Immunohistochemistry
Cell cultures were fixed with 4% paraformaldehyde at 4°C for 15 minutes, then permeabilized by incubation for 5 minutes in 0.1% Triton X-100 in PBS and incubated with 0.1% bovine serum albumin and 1% normal goat serum in PBS for 45 minutes. Cells were incubated with a rabbit polyclonal antibody to CD46 (Invitrogen-Gibco, Life Technologies) and a mouse monoclonal antibody to zonula occludens-1 (ZO-1; Invitrogen-Gibco, Life Technologies) overnight at 4°C. Cells were washed and incubated for 1 hour at 37°C in the dark with a goat anti-rabbit IgG antibody conjugated to Alexa Fluor 594 (red fluorescence; Invitrogen-Molecular Probes, Grand Island, NY, USA) and a goat anti-mouse IgG antibody conjugated to Alexa Fluor 488 (green fluorescence; Invitrogen-Molecular Probes). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) by incubating the cells in the dye solution for 10 minutes. The cells were then washed four times in PBS. Immunologically-stained cell cultures were visualized by a Zeiss LSM 510 NLO (Zeiss, Jena, Germany) confocal laser scanning microscope (Medical University of South Carolina Cell and Molecular Imaging Shared Resource) using a Plan-Apochromat 63X/1.4 oil DIC objective (Zeiss).
Western Blot Analyses of CD46 Expression
To detect expression of CD46 in ARPE-19 cells, cell suspensions were lysed and separated by electrophoresis on a 10% BisTris polyacrylamide gel (Invitrogen-Gibco, Life Technologies) with 10 μg protein loaded to each lane. Proteins were then transferred to a polyvinylidene difluoride membrane. The membrane was probed with a rabbit monoclonal antibody to CD46 (EPR4014; Abcam, Cambridge, MA, USA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cell Signaling Technology, Danvers, MA, USA) was used as a protein loading control. Both proteins were visualized by using chemiluminescence (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Density of the protein bands was accomplished by measuring band intensity with ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA). CD46 data were normalized to the total GAPDH control.
CD46 Silencer siRNA Transfection
ARPE-19 cells were cultured on 24-well inserts and grown to confluence for 7 days. CD46 or random siRNA were transfected into confluent ARPE-19 cells. Amine transfection agent (Ambion, Life Technologies, Grand Island, NY, USA) was used to transfect the siRNA oligos (Ambion, Life Technologies) into cultured ARPE-19 cells. Culture media from the basal (in 24-well plates) or apical (within inserts) side of ARPE-19 cultures was collected 48 hours post transfection. CD46 expression was determined by Western blot as described above, and VEGF was determined by ELISA using a human VEGF Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA), and results were analyzed by using a FLx 800 Microplate Reader (Biotek, Winooski, VT, USA) set at 450 nm.
VEGF Activation Assay on Nitrite-Modified Surfaces
Transwell permeable supports (Corning) in 12-well plates containing RPE-ECM were used in these experiments. ARPE-19 cells were plated onto one of four ECM conditions as described above. Cells were grown to confluence for 7 days in DMEM, 10% FBS, and antibiotics. ARPE-19 cell monolayer development and establishment of barrier function were assessed by monitoring transepithelial resistance by means of an epithelial volt/ohm meter using an electrode (STX2; World Precision Instruments, Sarasota, FL, USA) at 1 week. The resistance values (Ω*cm2) for the monolayers were determined from the average of four independent measurements, and resistance values were corrected for background resistance produced by the insert in the presence of RPE-derived ECM and medium. The resistance values for all groups ranged between 51 and 66 Ω*cm2, which are in the range of resistance for confluent ARPE-19 monolayers.39,40 Media was removed, replaced with serum-free media, and exposed to 25% complement-sufficient normal human serum (Atlanta Biologicals, Lawrenceville, GA, USA) applied to the basolateral surface (outside of permeable support) of cultured ARPE-19 monolayers for 4 hours. The supernatants were then collected and assayed for VEGF by using a human VEGF Quantikine ELISA Kit (R&D Systems). The results were analyzed by using a SpectraMax M5 Multi-Mode Microplate Reader (Molecular Devices, Inc., Sunnyvale, CA, USA) set at 450 nm. Aliquots were assayed in triplicate and values were compared with a VEGF165 standard dose-response curve.
Data Analyses
One-way ANOVA and t-tests were performed with Prism (GraphPad Software, Inc., La Jolla, CA, USA). A criterion of α = 0.05 was adopted. Each experiment was done in triplicate.
Results
CD46 Is Expressed Basolaterally in ARPE-19 Cells on RPE-ECM
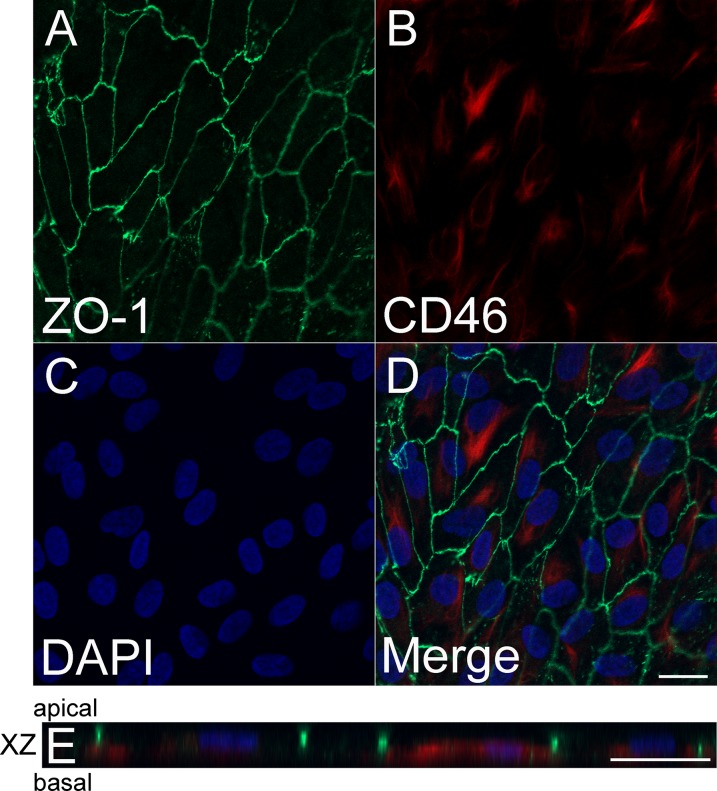
Confocal microscopy revealed positive ZO-1 expression at the cell margins of ARPE-19 cells (apical intercellular area) seeded on RPE-ECM (Fig. 1A). CD46 staining was present intracellularly and at the basolateral surface of ARPE-19 cells cultured on RPE-ECM (Figs. 1B, 1D). Nuclei were stained with DAPI (Fig. 1C). Microscopy through the XZ plane revealed apical expression of ZO-1 and basolateral and intracellular localization of CD46 (Fig. 1E).
Figure 1.
CD46 expression in RPE grown on RPE-derived ECM. Expression of ZO-1 at the cell margins of ARPE-19 cells (apical intercellular area) (A). There is abundant expression of CD46 intracellularly and at the basal margins of the cell (B), DAPI nuclear staining (C), and merged image (D). XZ projection shows apical expression of ZO-1 and basolateral and intracellular localization of CD46 (E). Scale bars: 20 μm. Green = anti–ZO-1, red = anti-CD46, blue = DAPI.
Extracellular Matrix Nitrite Modification Decreased CD46 Expression in ARPE-19
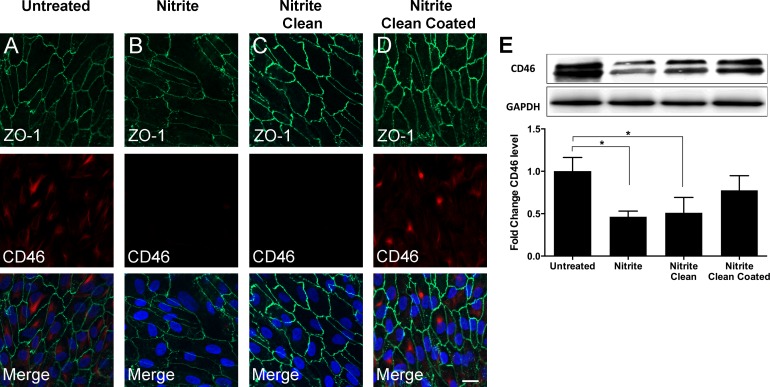
To model the aging effect on BM, we used a previously established approach of nitrite-modifying RPE-ECM.3 Immunohistochemistry and Western blot analysis showed that CD46 expression was greatest in ARPE-19 cells seeded onto untreated ECM (Fig. 2). This was significantly greater than that of ARPE-19 cells seeded onto nitrite-modified RPE-ECM (P = 0.003; Figs. 2A, 2B, 2E). ARPE-19 cells seeded onto nitrite-modified ECM, which was subsequently cleaned with Triton X-100 (nitrite-modified, cleaned treatment), expressed similar levels of CD46 as nitrite modified only, as measured by immunofluorescence (Figs. 2B, 2C). Western blot revealed slightly greater production of CD46 in ARPE-19 cells seeded onto nitrite-modified ECM that was subsequently cleaned with Triton X-100 than in cells on RPE-ECM that had been nitrite modified only, but this difference was not significant (P = 0.83; Fig. 2E). Coating previously nitrite-modified and cleaned ECM with extracellular proteins (nitrite-modified, cleaned, coated treatment) increased expression of CD46 in seeded ARPE-19 cells, such that levels were not significantly different from those of untreated ECM seeded ARPE-19 cells (fold change was −0.22, P = 0.32; Figs. 2A, 2D, 2E). Therefore, nitrite-modifying the surface of the ECM led to decreased levels of CD46 in ARPE-19 cells seeded onto this surface, relative to untreated ECM. This finding was reversed in ARPE-19 cells by cleaning nitrite from the ECM and coating with extracellular proteins.
Figure 2.
CD46 expression on nitrite-modified ECM. Immunofluorescence staining was positive for ZO-1 in all groups (A–D). Staining was positive for CD46 on untreated RPE-derived ECM (A). CD46 expression was reduced on nitrite-modified RPE-derived ECM and nitrite-modified ECM cleaned with Triton X-100 (B, C). CD46 expression was increased on nitrite-modified ECM cleaned with Triton X-100 and coated with ECM proteins (D). Quantification by Western blot analysis showed CD46 expression was significantly increased on untreated RPE-derived ECM as compared with nitrite-modified ECM and nitrite-modified ECM that had been cleaned with Triton X-100 (E). Control for (E) = GADPH. Scale bars: 20 μm (A–D). *P < 0.05. See Figure 1 for color legend.
siRNA Knockdown of CD46 in ARPE-19 Cells Leads to Increased VEGF Release
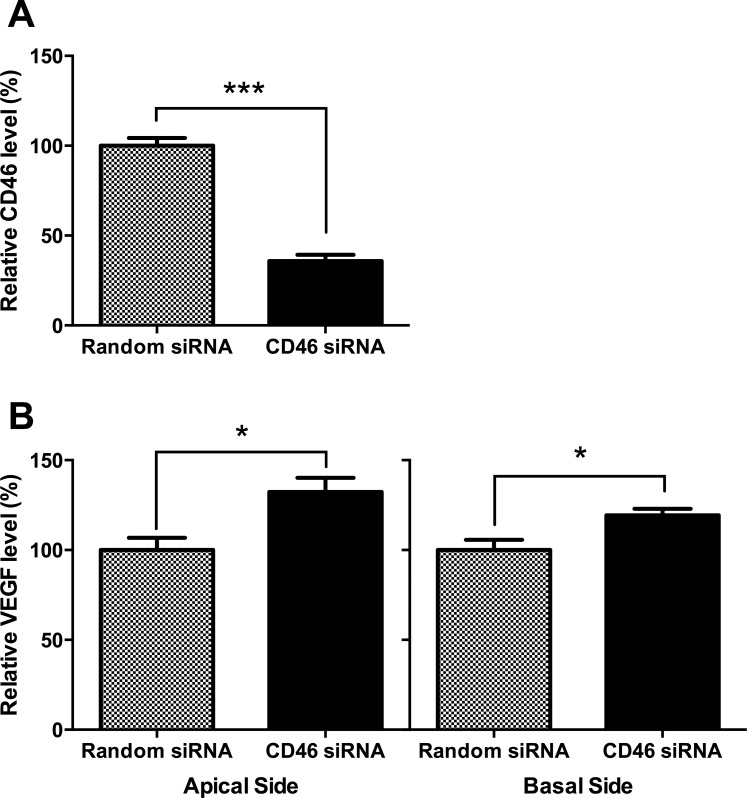
To investigate whether CD46 may play a functional role in VEGF activation, we knocked down CD46 gene expression via siRNA. CD46 expression was significantly decreased after treatment with CD46 siRNA (Fig. 3A). Following knockdown of CD46, VEGF release was significantly increased on the apical surface of ARPE-19 cells, relative to random siRNA, by 32% (P = 0.012; Fig. 3B, left panel). Similarly, VEGF release was significantly increased on the basolateral surface of ARPE-19 cells, relative to random siRNA, by 19% (P = 0.017; Fig. 3B, right panel).
Figure 3.
CD46 knockdown affected the release of VEGF in ARPE-19 cells. ARPE-19 cells were cultured until confluent and CD46 siRNA or random siRNA was transfected into these cells. Retinal pigment epithelial cells were collected after 48 hours of transfection. CD46 expression was decreased after treatment with CD46 siRNA (A). CD46 knockdown in ARPE-19 cells also increased the release of VEGF (B). *P < 0.05, ***P < 0.001.
The Effect of ECM Nitrite Modification on VEGF Activation in ARPE-19 Cells
We investigated VEGF release in ARPE-19 cells seeded onto a similar system to that mentioned above. However, this time RPE-ECM was cultured onto Transwell permeable supports and basally exposed to 25% normal human serum. Vascular endothelial growth factor release was quantified in media from both the apical and basal surfaces of ARPE-19 cells by ELISA.
Apical.
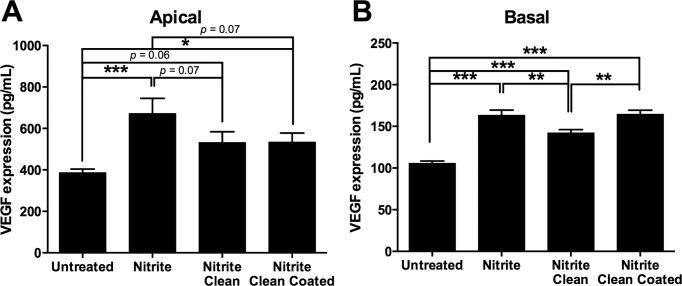
The apical release of VEGF was lowest in ARPE-19 cells seeded onto untreated ECM (Fig. 4A). Nitrite modification of the surface increased the release of VEGF apically by 286 pg/mL (P < 0.001; Fig. 4A). ARPE-19 cells seeded onto nitrite-modified ECM, which was subsequently cleaned with Triton X-100 (nitrite-modified, cleaned treatment), expressed less VEGF apically than cells on RPE-ECM that had been nitrite modified only, although this did not reach significance (−140 pg/mL difference, P = 0.07; Fig. 4A). Both nitrite-modified and cleaned, as well as nitrite-modified, cleaned, and coated treatments, had a similar effect on ARPE-19 cells (P = 0.98), whereby both treatments led to a decrease in VEGF release by −134 pg/mL (P = 0.071) and −142 pg/mL (P = 0.068), respectively, relative to nitrite modified only. Additionally, there was a moderate and significant difference between VEGF release in cells seeded onto cleaned ECM, coated with ECM proteins (nitrite-modified, cleaned, coated treatment), and that of untreated ECM seeded ARPE-19 cells (mean difference was 148 pg/mL, P = 0.05; Fig. 4A). Thus, nitrite modification of the ECM surface led to increased levels of VEGF in ARPE-19 cells relative to untreated ECM.
Figure 4.
Nitrite modification of the ECM affects VEGF released after exposure to normal human serum. ARPE-19 cells were cultured and the basolateral surface exposed to normal human serum on nitrite-modified ECM, nitrite-modified ECM cleaned with Triton X-100, nitrite-modified ECM cleaned with Triton X-100 and coated with ECM proteins, or untreated RPE-derived ECM as the control. Apical VEGF levels were elevated in nitrite-modified ECM exposed to normal human serum and significantly higher than in untreated RPE-derived ECM (A). Basal VEGF levels were also elevated on nitrite-modified ECM compared with untreated RPE-derived ECM (B). *P < 0.05, **P < 0.01, ***P < 0.001.
Basal.
The basal release of VEGF was lowest in the media from ARPE-19 cells seeded onto untreated ECM (Fig. 4B). Nitrite modification of the surface increased basal release of VEGF by 58 pg/mL (P < 0.001; Fig. 4B). Cleaning the nitrite-modified surface with Triton X-100 (nitrite-modified, cleaned treatment) decreased VEGF relative to nitrite-modified ECM (P = 0.004; Fig. 4B). Interestingly, there was a significant increase between VEGF release by cells seeded onto cleaned ECM that were nitrite modified, cleaned and coated, compared with those on ECM that were nitrite modified and cleaned only (increase was 22 pg/mL, P = 0.003; Fig. 4B). Additionally, there was a highly significant increase in VEGF activation from cells on ECM that were nitrite modified, cleaned, and coated, relative to those on untreated ECM (increase was 59 pg/mL, P < 0.001; Fig. 4B). Thus, nitrite-modifying the surface of ECM led to increased levels of VEGF release by ARPE-19 cells seeded onto this surface, relative to those in untreated ECM. Subsequent cleaning of nitrite led to a trend toward restoring normal levels of VEGF. However, the addition of ECM proteins to previously nitrite-modified and cleaned ECM led to greater activation of VEGF levels than with those that were nitrite modified and cleaned only. Additionally, the overall pattern of responses across all ECM treatments was similar for both the apical and basal sides of the RPE.
Discussion
CD46 is a complement regulatory protein that plays a major role in regulating the inflammatory response.35 While the association between CD46 and RPE atrophy still remains to be fully elucidated, this protein appears to play a critical role in RPE cell homeostasis on human BM.32 A decrease in CD46 expression is observed in the RPE of patients with GA, and this decrease is progressive.33 Thus, CD46 may be a critical component of the healthy RPE phenotype, and loss of CD46 may play a role in disease pathogenesis.
Herein, we investigated the expression of CD46 on nitrite-modified ECM derived from RPE cells, an in vitro model of “aging.”19 We have previously demonstrated that nitrite modification of RPE-ECM has a significant effect on altering RPE attachment, proliferation, and survival.3 Additionally, we have previously demonstrated that nitrite modification of RPE-ECM has a detrimental effect on important RPE functions such as phagocytosis.20
CD46 is expressed on the basolateral surface of polarized epithelium and is a critical component of cellular adhesion to ECM.32,41–44 In the current study, we demonstrated that there is polarized localization of CD46 in ARPE-19 cells when these cells are cultured onto a RPE-derived ECM. The basolateral polarization of CD46 supports the notion that this molecule protects the cell from complement-mediated damage, and its location suggests a role in maintaining a healthy RPE phenotype by contributing to cell adhesion to the basement membrane.32,41
Treatment with anti-CD46 antibody (blocking antibody) leads to a dose-dependent inhibition of cell attachment to the basal lamina of human BM.32 It has been observed that alterations in RPE-CD46 expression progressively increase as RPE morphology deteriorates in the transition from healthy to a GA phenotype.33 Changes in CD46 expression are even seen during the early course of this disease, at a time when RPE cells are at near normal morphology.33 This suggests that the protein's association with RPE cells may form the functional units underlying normal adhesion mechanisms, maintaining a healthy RPE phenotype that is not proliferative or migratory.32 Indeed, recently it has been shown that CD46−/− mice are more susceptible to experimental CNV owing to insufficient complement inhibition and increased VEGF.28 Moreover, it has been shown that expression of human CD46 by adenovirus infection reduces complement-mediated damage on murine ocular flat-mounts after challenge with human serum, suggesting that the protein may play a protective role in the retina during disease pathogenesis such as AMD.45
In the current study, we demonstrated that culturing RPE cells on nitrite-modified RPE-ECM significantly reduced CD46 expression when compared to untreated RPE-ECM. This reduction in CD46 was reversed on nitrite-modified ECM that is subsequently cleaned and/or coated with extracellular proteins. It has been demonstrated that CD46, along with β1-integrin, plays an important role in RPE cell adhesion to basement membrane, and both bear a close association to one another.32 Aging and/or disease progression of BM reduces the ability of both proteins to adhere to ECM. Loss of these proteins may lead to the progression of AMD and make RPE cells more susceptible to complement exposure and eventual damage, as seen in both wet and dry AMD.
The direct role of CD46 expression on VEGF release was confirmed by siRNA treatment of ARPE-19 cells. Reduced expression of CD46 using targeted siRNA resulted in increased VEGF release by RPE cells, suggesting a regulatory role for CD46 in VEGF release by RPE cells. Nitrite modification of ECM reduced CD46 expression and increased apical and basal VEGF release by ARPE-19 cells. To our knowledge, this is the first direct demonstration that age-related modification of the basement membrane leads to a change in both CD46 expression and VEGF release. It is difficult to not overemphasize the importance of the observation that changes of the basement membrane due to aging will decrease CD46 expression, because CD46 is an important inhibitor of the complement cascade; and reduced expression of CD46, which is located on the basolateral cell surface, will expose the RPE to activated complement components present in serum. This exposure could potentially exacerbate disease.46,47 Our data are consistent with prior studies showing that complement activation from oxidative stress, induced by exposing RPE cells to H2O2 and normal human serum, will increase VEGF secretion.48 Our data are also consistent with the observation that CD46 knockout mice demonstrate an increased VEGF production in the eye and develop more severe disease in a laser-induced model of CNV.28
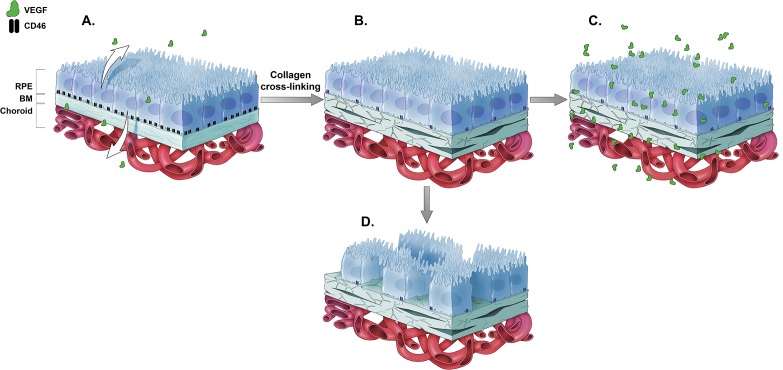
The relationship between aging in BM, CD46 expression, atrophy, and release of VEGF from the RPE is summarized in Figure 5. In the normal human eye, CD46 is expressed on the basolateral surface of the RPE and is closely associated with the β1-integrin subunit involved in cell adhesion. Vascular endothelial growth factor is released apically and basally by the native, healthy RPE (Fig. 5A). Collagen cross-linking occurs in human eyes as a function of age, or can be induced in tissue culture by nitrite modification of the basement membrane. This reduces the expression of CD46 on the basement membrane of the RPE, which makes the cell more susceptible to complement-mediated damage (Fig. 5B). These age-related changes in basement membrane also increase the amount of VEGF that is released into the extracellular milieu, with an increase in both apical and basal VEGF release (Fig. 5C). In addition, the basement membrane changes seen early in the course of AMD will also lead to changes in VEGF that can enhance either CNV (basal VEGF release) or induce retinal angioma formation (apical VEGF release).49 Alternatively, progressive loss of CD46 may contribute to atrophy as seen in advanced GA (Fig. 5D).
Figure 5.
Effects of nitrite exposure on CD46 and VEGF on RPE cells. Young (A) and aged (B) RPE cells are diagrammatically represented here. CD46 was expressed on the basolateral surface of the RPE and played a role in RPE cell adhesion to the basement membrane. Cross-linking of collagen in Bruch's membrane (through exposure to nitrite) reduced the expression of CD46 on the RPE. This reduction led to increased release of VEGF (C). CD46 also contributed to successful RPE cell adhesion to the basement membrane, and progressive loss of CD46 may contribute to atrophy (D). Thus, it is expected that loss of CD46 in aged or diseased Bruch's membrane increased VEGF production in the RPE and potentially contributed to the pathology seen in exudative AMD. Loss of CD46 alternatively contributed to the loss of RPE, resulting in the pathology seen in advanced AMD or geographic atrophy.
The surprising observations that cleaning with the addition of ECM protein did not reduce VEGF release basally may be due to differing effects of individual ECM proteins on the behavior of epithelial and endothelial cells. For example, laminin increases VEGF release and promotes differentiation of endothelial cells.50 Studies conducted on RPE cell cultures have shown that the addition of soluble matrix proteins results in moderate increases in VEGF expression in an experimental model of hypoxia.51 Moreover, it has been shown that culturing of endothelial cells on different matrices such as vitronectin can increase expression of VEGF receptor.52 These observations may explain the increase in basolateral VEGF release upon the addition of ECM proteins. Alternatively, increased basal VEGF production in these groups could possibly rest on the fact that neither cleaning nor cleaning and coating with matrix protein reverse the nitrate-induced protein cross-linking, making it more difficult to alter behavior of these cells. Alternate use of cross-link breakers could be examined in this model of aging. More investigation is needed to determine the molecular mechanisms responsible for this effect.
Numerous changes develop within human BM as a function of age, including the accumulation of drusen between the RPE and basement membrane, basal laminar, and basal linear deposits,4,5 collagen cross-linking in the inner and outer collagen layer, calcification and fragmentation of the elastin layer,6 and membrane lipidization.6–8 Given the complexity of these anatomic changes, it is important that in vitro models of BM pathology be developed to investigate the connection between basement membrane changes and disease pathogenesis. The use of nitrite-modified surfaces to mimic the effects of inflammation and aging serves as a relevant model to study the progression of diseases such as AMD.20 Nitrite exposure, as seen during chronic inflammation from aging and cigarette smoking, can directly modify ECM proteins and have a damaging effect on ECM structure.3,53 Thus, this exposure can lead to the structural damage associated with the cross-linking of proteins in the BM, one of the hallmark changes associated with basement membrane aging and disease. Nitrite modification of the ECM has been used previously as a model of basement membrane aging in AMD.19,20
In conclusion, this study demonstrated that nitrite modification of RPE-derived basement membrane reduced CD46 expression in RPE cultured onto this surface. This leads to a downregulation of CD46, which places the cell at higher risk for apical and basal release of VEGF from the RPE. This can lead to neovascularization manifested as CNV or retinal angiomatous proliferation. Our results suggest a direct connection between the basement membrane changes seen in AMD and the cellular changes (atrophy and neovascularization) that are seen later in disease.
Acknowledgments
Supported in part by an unrestricted grant to the Medical University of South Carolina, Department of Ophthalmology, from Research to Prevent Blindness (New York, NY, USA) and the Foundation Fighting Blindness (Columbia, MD, USA). Also supported in part by the Cell & Molecular Imaging Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313). The authors thank Bärbel Rohrer, PhD, and Rosalie Crouch, PhD, for their contributions to this manuscript. The authors alone are responsible for the content and writing of the paper.
Disclosure: M.A. Fields, None; H. Cai, None; H.E. Bowrey, None; E.F. Moreira, None; M. Beck Gooz, None; K. Kunchithapautham, None; J. Gong, None; E. Vought, None; L.V. Del Priore, None
References
- 1. Friedman DS,, O'Colmain BJ,, Munoz B,, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122: 564–572. [DOI] [PubMed] [Google Scholar]
- 2. Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976; 60: 324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Z,, Paik DC,, Del Priore LV,, Burch RL,, Gaillard ER. Nitrite-modified extracellular matrix proteins deleteriously affect retinal pigment epithelial cell function and viability: a comparison study with nonenzymatic glycation mechanisms. Curr Eye Res. 2005; 30: 691–702. [DOI] [PubMed] [Google Scholar]
- 4. Pauleikhoff D,, Harper CA,, Marshall J,, Bird AC. Aging changes in Bruch's membrane: a histochemical and morphologic study. Ophthalmology. 1990; 97: 171–178. [PubMed] [Google Scholar]
- 5. Sarks SH,, Arnold JJ,, Killingsworth MC,, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age-related maculopathy: a clinicopathological study. Br J Ophthalmol. 1999; 83: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spraul CW,, Lang GE,, Grossniklaus HE,, Lang GK. Histologic and morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999; 44( suppl 1): S10–32S. [DOI] [PubMed] [Google Scholar]
- 7. Abdelsalam A,, Del Priore L,, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999; 44: 1–29. [DOI] [PubMed] [Google Scholar]
- 8. Mullins RF,, Aptsiauri N,, Hageman GS. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye. 2001; 15: 390–395. [DOI] [PubMed] [Google Scholar]
- 9. Marshall GE,, Konstas AG,, Reid GG,, Edwards JG,, Lee WR. Type IV collagen and laminin in Bruch's membrane and basal linear deposit in the human macula. Br J Ophthalmol. 1992; 76: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chong NH,, Keonin J,, Luthert PJ,, et al. Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am J Pathol. 2005; 166: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mullins RF,, Russell SR,, Anderson DH,, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000; 14: 835–846. [PubMed] [Google Scholar]
- 12. Crabb JW,, Miyagi M,, Gu X,, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002; 99: 14682–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hageman GS,, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20: 705–732. [DOI] [PubMed] [Google Scholar]
- 14. Johnson LV,, Leitner WP,, Rivest AJ,, Staples MK,, Radeke MJ,, Anderson DH. The Alzheimer's Aβ-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002; 99: 11830–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murdaugh LS,, Wang Z,, Del Priore LV,, Dillon J,, Gaillard ER. Age-related accumulation of 3-nitrotyrosine and nitro-A2E in human Bruch's membrane. Exp Eye Res. 2010; 90: 564–571. [DOI] [PubMed] [Google Scholar]
- 16. Karwatowski WS,, Jeffries TE,, Duance VC,, Albon J,, Bailey AJ,, Easty DL. Preparation of Bruch's membrane and analysis of the age-related changes in the structural collagens. Br J Ophthalmol. 1995; 79: 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solberg Y,, Rosner M,, Belkin M. The association between cigarette smoking and ocular diseases. Surv Ophthalmol. 1998; 42: 535–547. [DOI] [PubMed] [Google Scholar]
- 18. Borland C,, Higenbottam T. Nitric oxide yields of contemporary UK, US and French cigarettes. Int J Epidemiol. 1987; 16: 31–34. [DOI] [PubMed] [Google Scholar]
- 19. Paik DC,, Dillon J,, Galicia E,, Tilson MD. The nitrite/collagen reaction: non-enzymatic nitration as a model system for age-related damage. Connective Tissue Res. 2001; 42: 111–122. [DOI] [PubMed] [Google Scholar]
- 20. Sun K,, Cai H,, Tezel TH,, Paik D,, Gaillard ER,, Del Priore LV. Bruch's membrane aging decreases phagocytosis of outer segments by retinal pigment epithelium. Mol Vis. 2007; 13: 2310–2319. [PubMed] [Google Scholar]
- 21. Anderson DH,, Mullins RF,, Hageman GS,, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134: 411–431. [DOI] [PubMed] [Google Scholar]
- 22. Wang L,, Clark ME,, Crossman DK,, et al. Abundant lipid and protein components of drusen. PLoS One. 2010; 5: e10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson DH,, Radeke MJ,, Gallo NB,, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010; 29: 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nozaki M,, Raisler BJ,, Sakurai E,, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006; 103: 2328–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thurman JM,, Renner B,, Kunchithapautham K,, et al. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009; 284: 16939–16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rohrer B,, Long Q,, Coughlin B,, et al. A targeted inhibitor of the complement alternative pathway reduces RPE injury and angiogenesis in models of age-related macular degeneration. Adv Exp Med Biol. 2010; 703: 137–149. [DOI] [PubMed] [Google Scholar]
- 27. Bora NS,, Kaliappan S,, Jha P,, et al. CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J Immunol. 2007; 178: 1783–1790. [DOI] [PubMed] [Google Scholar]
- 28. Lyzogubov V,, Wu X,, Jha P,, et al. Complement regulatory protein CD46 protects against choroidal neovascularization in mice. Am J Pathol. 2014; 184: 2537–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bora NS,, Jha P,, Lyzogubov VV,, et al. Recombinant membrane-targeted form of CD59 inhibits the growth of choroidal neovascular complex in mice. J Biol Chem. 2010; 285: 33826–33833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fremeaux-Bacchi V,, Sanlaville D,, Menouer S,, et al. Unusual clinical severity of complement membrane cofactor protein-associated hemolytic-uremic syndrome and uniparental isodisomy. Am J Kidney Dis. 2007; 49: 323–329. [DOI] [PubMed] [Google Scholar]
- 31. Richards A,, Kathryn Liszewski M,, Kavanagh D,, et al. Implications of the initial mutations in membrane cofactor protein (MCP; CD46) leading to atypical hemolytic uremic syndrome. Mol Immunol. 2007; 44: 111–122. [DOI] [PubMed] [Google Scholar]
- 32. McLaughlin BJ,, Fan W,, Zheng JJ,, et al. Novel role for a complement regulatory protein (CD46) in retinal pigment epithelial adhesion. Invest Ophthalmol Vis Sci. 2003; 44: 3669–3674. [DOI] [PubMed] [Google Scholar]
- 33. Vogt SD,, Curcio CA,, Wang L,, et al. Retinal pigment epithelial expression of complement regulator CD46 is altered early in the course of geographic atrophy. Exp Eye Res. 2011; 93: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson LV,, Leitner WP,, Staples MK,, Anderson DH. Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp Eye Res. 2001; 73: 887–896. [DOI] [PubMed] [Google Scholar]
- 35. Barilla-LaBarca ML,, Liszewski MK,, Lambris JD,, Hourcade D,, Atkinson JP. Role of membrane cofactor protein (CD46) in regulation of C4b and C3b deposited on cells. J Immunol. 2002; 168: 6298–6304. [DOI] [PubMed] [Google Scholar]
- 36. Seya T,, Atkinson JP. Functional properties of membrane cofactor protein of complement. Biochem J. 1989; 264: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liszewski MK,, Farries TC,, Lublin DM,, Rooney IA,, Atkinson JP. Control of the complement system. Adv Immunol. 1996; 61: 201–283. [DOI] [PubMed] [Google Scholar]
- 38. Liszewski MK,, Post TW,, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991; 9: 431–455. [DOI] [PubMed] [Google Scholar]
- 39. Ablonczy Z,, Dahrouj M,, Tang PH,, et al. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci. 2011; 52: 8614–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dunn KC,, Aotaki-Keen AE,, Putkey FR,, Hjelmeland LM. ARPE-19 a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996; 62: 155–169. [DOI] [PubMed] [Google Scholar]
- 41. Maisner A,, Liszewski MK,, Atkinson JP,, Schwartz-Albiez R,, Herrler G. Two different cytoplasmic tails direct isoforms of the membrane cofactor protein (CD46) to the basolateral surface of Madin-Darby canine kidney cells. J Biol Chem. 1996; 271: 18853–18858. [DOI] [PubMed] [Google Scholar]
- 42. Fett AL,, Hermann MM,, Muether PS,, Kirchhof B,, Fauser S. Immunohistochemical localization of complement regulatory proteins in the human retina. Histol Histopathol. 2012; 27: 357–364. [DOI] [PubMed] [Google Scholar]
- 43. Maisner A,, Zimmer G,, Liszewski MK,, et al. Membrane cofactor protein (CD46) is a basolateral protein that is not endocytosed: importance of the tetrapeptide FTSL at the carboxyl terminus. J Biol Chem. 1997; 272: 20793–20799. [DOI] [PubMed] [Google Scholar]
- 44. Teuchert M,, Maisner A,, Herrler G. Importance of the carboxyl-terminal FTSL motif of membrane cofactor protein for basolateral sorting and endocytosis: positive and negative modulation by signals inside and outside the cytoplasmic tail. J Biol Chem. 1999; 274: 19979–19984. [DOI] [PubMed] [Google Scholar]
- 45. Sweigard JH,, Cashman SM,, Kumar-Singh R. Adenovirus-mediated delivery of CD46 attenuates the alternative complement pathway on RPE: implications for age-related macular degeneration. Gene Ther. 2011; 18: 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kunchithapautham K,, Atkinson C,, Rohrer B. Smoke exposure causes endoplasmic reticulum stress and lipid accumulation in retinal pigment epithelium through oxidative stress and complement activation. J Biol Chem. 2014; 289: 14534–14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scholl HP,, Charbel Issa P,, Walier M,, et al. Systemic complement activation in age-related macular degeneration. PLoS One. 2008; 3: e2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bandyopadhyay M,, Rohrer B. Matrix metalloproteinase activity creates pro-angiogenic environment in primary human retinal pigment epithelial cells exposed to complement. Invest Ophthalmol Vis Sci. 2012; 53: 1953–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yannuzzi LA,, Negrao S,, Iida T,, et al. Retinal angiomatous proliferation in age-related macular degeneration. 2001. Retina. 2012; 32 (suppl 1): 416–434. [DOI] [PubMed] [Google Scholar]
- 50. Dixelius J,, Jakobsson L,, Genersch E,, Bohman S,, Ekblom P,, Claesson-Welsh L. Laminin-1 promotes angiogenesis in synergy with fibroblast growth factor by distinct regulation of the gene and protein expression profile in endothelial cells. J Biol Chem. 2004; 279: 23766–23772. [DOI] [PubMed] [Google Scholar]
- 51. Mousa SA,, Lorelli W,, Campochiaro PA. Role of hypoxia and extracellular matrix-integrin binding in the modulation of angiogenic growth factors secretion by retinal pigmented epithelial cells. J Cell Biochem. 1999; 74: 135–143. [PubMed] [Google Scholar]
- 52. Tsou R,, Isik FF. Integrin activation is required for VEGF and FGF receptor protein presence on human microvascular endothelial cells. Mol Cell Biochem. 2001; 224: 81–89. [DOI] [PubMed] [Google Scholar]
- 53. Capuron L,, Schroecksnadel S,, Feart C,, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011; 70: 175–182. [DOI] [PubMed] [Google Scholar]