Abstract
Rice husk is a potential source for renewable energy and silica. To extract the maximum amount of silica, usually the rice husk is treated with strong acids that burn the organic part leaving behind a black residue. In this research, sulfuric acid is used as an oxidizing agent. Efforts are focused to find out more about the behavior of acid-treated rice husk by using thermal exposure, and results are compared with results for raw rice husk which is thermally exposed but not acid treated. Reaction ratio of rice husk combustion and energy of activation were calculated using the thermogravimetric data. Acid treatment was found influential in initiating degradation earlier compared to raw husk and an overall increase in value of activation energy was observed when heating rate was increased.
Keywords: Acid leaching, Thermal kinetics, Rice husk, Flynn and Wall expression
Introduction
Rice husk (RH) is an agro-industrial by-product of the rice milling process and is used for a number of traditional and technical applications. In addition to organic matter, RH contains about 15–25% of micrometer size silica particles [1], [2] which are naturally embedded in the cellulosic part of rice husk [3], [4]. Complete combustion of rice husk produces rice husk ash (RHA) that contains about 95% of silica and traces of metallic oxides [5]. Traditional applications of rice husk include their use as low burning fuel, soil conditioner and cardboard material. In modern-day applications, it is being used for synthesis of pure silicon [6], [7], [8] and various silicon based materials, viz. silica nanoparticles [9], [10], [11], silicon carbide [12], [13], [14], [15], [16], [17], [18], [19], silicon nitride, silicon oxynitride [20], [21], [22], [23], silicon tetrachloride [24], [25], [26], [27] and zeolites [28], [29], [30], [31]. Other uses include: fuel in power plants; use in the production of activated carbon, porous silica/carbon composites, insulating fire bricks and various organic compounds (xylitol, furfural, ethanol, acetic acid, lingo sulfonic acid) [32]. Rice husk is subjected to higher temperatures during the synthesis of most of these products and therefore its thermal kinetics are always of interest in order to optimize process parameters and product yield. Response of rice husk to thermal exposure has been studied through thermogravimetric analysis (TGA) under different atmospheres. Mansary and Ghaly explored the thermal kinetics of rice husk under air, nitrogen and argon atmospheres [33], [34], [35], [36], [37]. Energy of activation is usually calculated using the Arrhenius equation. Another convenient method employs the Flynn and Wall expression [38] which eliminates calculations for rate constant and pre-exponential factor. However, as it includes heating rate, the Flynn and Wall expression requires the TGA to be conducted under non-isothermal conditions. The type of purge gas used, the heating rate and the particle size of the sample used for thermal analysis all affect the thermal kinetics of rice husk. Another factor affecting thermal behavior of rice husk is the pre-treatment applied. Rice husk, prior to synthesis or thermal analysis, can be treated with various reagents or catalysts such as a mineral acid [39], an alkali [40], [42] or sodium silicate [3]. The present work deals with the effect of acid leaching on the thermal kinetics of rice husk and explores the use of sulfuric acid for leaching of rice husk to obtain silica. If the concentration and heating rate are controlled properly, it is possible to get low cost silica from rice husk in a very short time.
Material and methods
Raw rice husk was procured from a local rice milling plant and rigorously rinsed with distilled water to remove any soil particles and residual rice grains. After rinsing, rice husk was subjected to acid treatment by soaking it in 5–6 N sulfuric acid solution for one and half hours with gentle stirring. Acid-treated rice husk was again washed with distilled water, pulverized to a particle size down to −100 mesh by means of ASTM standard sieving and stored in a drying oven at 80 °C. Thermogravimetric analysis and differential thermal analysis (TGA and DTA) of acid-treated rice husk were carried out using LINSIES PT1600 thermal analyser. Samples of 10 mg weight were heated in a nitrogen atmosphere from ambient to 800 °C at heating rates 5, 10 and 20 °C/min. The reaction ratio of combustion (Rc) was determined by using the following expression [43]:
All these mass values were carefully taken from TG curves. TG curves were also used to draw isoconversional curves to explore the kinetics of rice husk thermal degradation from 10% to 60% mass loss. Calculations for energy of activation (Ea) were based on the Flynn and Wall expression [5]:
where R is molar gas constant, β is heating rate and T is the absolute temperature.
Results and discussion
Differential thermal analysis
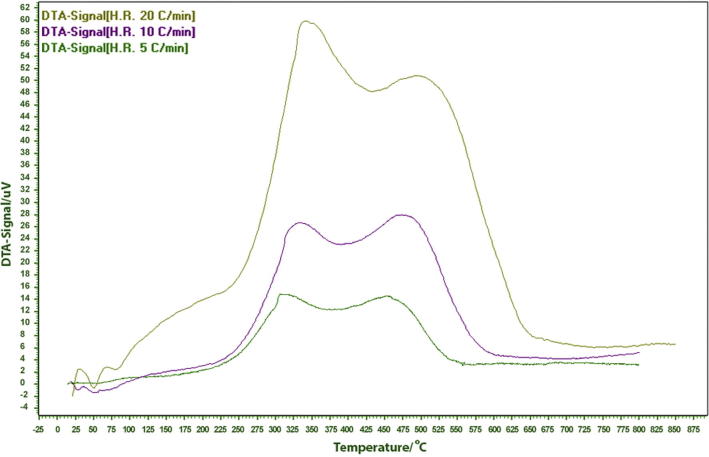
Acid leaching removes metallic impurities from rice husk which are present in oxides form [38]. Fig. 1 shows DTA curves of acid-treated rice husk obtained at different heating rates. Exothermic peaks at 300–325 °C correspond to decomposition of organic matter whereas those at around 450–475 °C show degradation of the cellulosic part of rice husk. Raw rice husk undergoes early decomposition at around 370 °C [1]. The influence of heating rate on the intensity of exothermic effect is also apparent.
Fig. 1.
DTA curves at heating rates of 5, 10 and 20 °C min−1.
Thermogravimetric analysis (TGA)
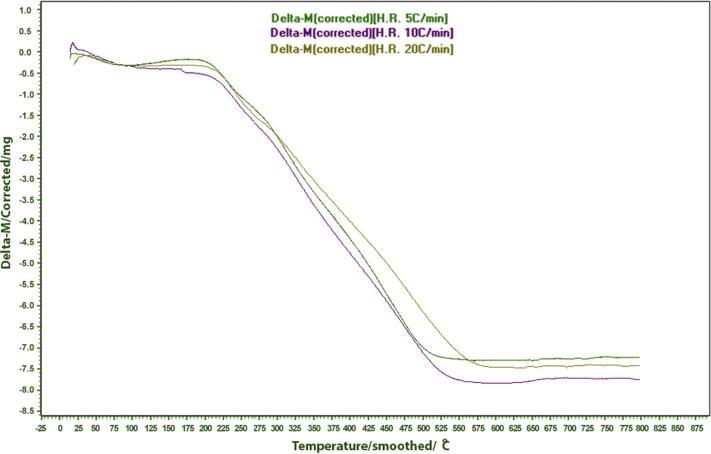
Rice husk is generally thermogravimetrically analyzed under non-isothermal conditions which make it possible to explore thermal kinetics over a continuous range of temperatures. Thermogravimetric curves of rice husk, shown in Fig. 2, provide a comparison on the basis of heating rate. The initial descending slant from the start of the curve to about 100 °C corresponds to loss of hygroscopic water. There is no considerable mass loss up to about 200 °C which shows the thermal stability of the organic constituents of the rice husk. It also indicates the good heating capability of rice husk when used as a low burning fuel. Mass loss from 200 to 550 °C can be divided into two parts. Mass loss in the range 230–330 °C was due to thermal decomposition and volatilization of the organic part of the rice husk, whereas the mass loss from 330 to 550 °C was due to the oxidation and gasification of the char (carbon). These two stages are usually termed as active pyrolysis zone and passive zone respectively. Thermal decomposition of raw rice husk starts at about 230 °C [33], [42], [44], [41] which is quite late compared to acid-treated rice husk (200 °C). Moreover, the acid-treated rice husk underwent a greater mass loss. In case of acid-treated rice husk, commencement of thermal decomposition at lower temperature can be ascribed to two factors: (i) acid leaching of partially oxidized carbohydrates and (ii) activated amide groups in rice husk such as NH2 and CN [20]. An increase in heating rate caused earlier instigation of thermal degradation which ultimately resulted in an earlier completion of mass loss phenomenon. In other words, an increase in heating rate resulted in a decrease in the initial degradation temperature.
Fig. 2.
Thermal gravimetric curves at heating rates of 5, 10 and 20 °C min−1.
Thermal degradation
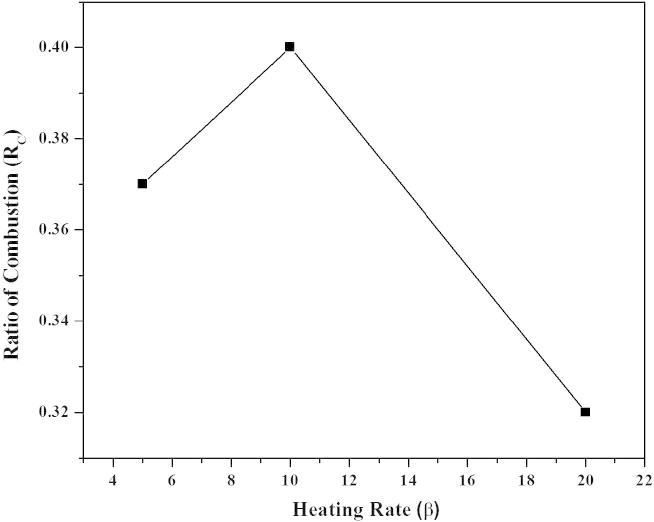
Since the rate of thermal degradation generally increases with increasing heating rate, the latter also affects the reaction ratio of combustion (Rc). Fig. 3 shows an overall inverse relation between heating rate and reaction ratio of combustion. The rate of thermal degradation increases with increasing activity and ionization of acid. The acid attack removes the volatile materials like water and other organic compounds from the cellulose (main part of rice husk). The residue left turns black because it now consists of only free carbon which is black.
Fig. 3.
Ratio of combustion (Rc)as a function of heating rate.
Activation energy
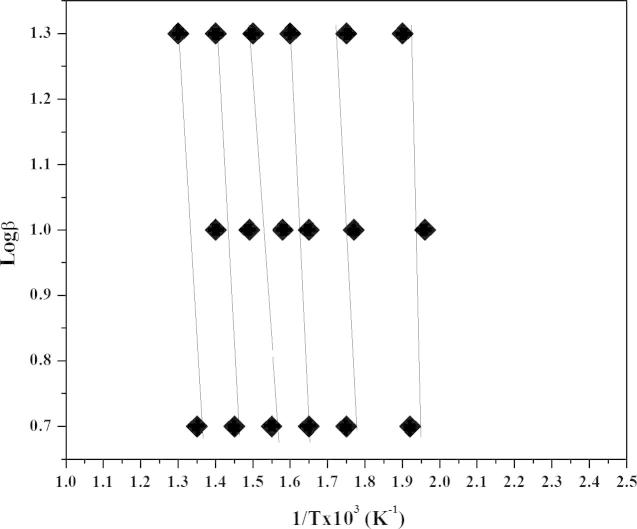
Energy of activation was calculated over a continuous range of mass losses resulting from the thermal decompositions. Mass losses from 10% to 60% with mass fractions α = 0.1 to 0.6 were considered (Table 1). Six straight lines were drawn, each corresponding to a specific degradation interval, taking 1/T at x/axis and log β at y/axis (Fig. 4). The slope of each line was used in the Flynn and Wall expression to determine the value of energy of activation for the corresponding degradation regions given in Table 2. An overall increase in Ea value is evident as degradation proceeded [5], [42]. An abrupt increase in Ea value comes after about 50% mass loss which confirms the completion of thermal degradation and volatilization of the organic part of rice husk after this stage.
Table 1.
Relationship between log β and 1/T from α = 0.1 to α = 0.6.
| Log β | 1/T × 103 K−1 |
|||||
|---|---|---|---|---|---|---|
| α = 0.1 | α = 0.2 | α = 0.3 | α = 0.4 | α = 0.5 | α = 0.6 | |
| 0.698 | 1.926 | 1.744 | 1.638 | 1.526 | 1.438 | 1.362 |
| 1.000 | 1.968 | 1.785 | 1.666 | 1.526 | 1.461 | 1.373 |
| 1.301 | 1.941 | 1.744 | 1.604 | 1.485 | 1.382 | 1.293 |
Fig. 4.
Isoconversional curves for rice husk (RH) by Flynn and Wall expression.
Table 2.
Linear expressions of isoconversional lines and corresponding values of Ea at different degradation intervals.
| α | Equation of straight line | Ea (kJ mol−1) |
|---|---|---|
| 0.1 | Y = −0.0447X + 1.99 | 0.814 |
| 0.2 | Y = −0.0679X + 1.80 | 1.235 |
| 0.3 | Y = −0.0563X + 1.69 | 1.024 |
| 0.4 | Y = −0.0679X + 1.60 | 2.235 |
| 0.5 | Y = −0.0928X + 1.47 | 1.688 |
Conclusions
Acid treatment of rice husk resulted in an effective partial oxidization of the carbohydrates and yielded a black residue material. A faster heating rate caused an early start of thermal degradation and consequently led to a faster degradation rate up to about 50% mass loss. After 50% mass loss, degradation rate decreased because all the organic matter had already been decomposed leaving a char residue. Acid treatment also caused a decrease in the energy of activation required to initiate thermal decomposition.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Padhi B.K., Patnaik C. Development of Si2N2O, Si3N4 and SiC ceramic materials using rice husks. Ceram Int. 1995;21:213–220. [Google Scholar]
- 2.Muthadi A., Anitha R., Kothandaraman S. Rice husk ash – properties and its uses; a review. IE(I)J – CV. 2007;88:50–56. [Google Scholar]
- 3.Janghorban K., Tazesh H.R. Effect of catalyst and process parameters on the production of silicon carbide from rice hulls. Ceram Int. 1997;25:7–12. [Google Scholar]
- 4.Chen X.-G., Lv, Zhang P.-P., Zhang L., Ye Y. Thermal destruction of rice hull in air and nitrogen. J Therm Anal Calorim. 2011;104:1055–1062. [Google Scholar]
- 5.Kim H.J., Eom Y.G. Thermogravimetric analysis of rice husk flour for a new raw material of lignocellulosic fibre-thermoplastic polymer composite. Mokchae Konghak J Korean Wood Sci Technol. 2001;29:59–67. [Google Scholar]
- 6.Banerjee H.D., Sen S., Acharya H.N. Investigations on the production of silicon from rice husks by magnesium method. Mater Sci Eng. 1982;52:173–179. [Google Scholar]
- 7.Bose D.N., Govinda P.A., Banerjee H.D. Large grain polycrystalline silicon from rice husks. Sol Energy Mater. 1982;7:319–321. [Google Scholar]
- 8.Okutani T. Utilization of silica in rice hulls as raw materials for silicon semiconductors. J Met Mater Min. 2009;19:51–59. [Google Scholar]
- 9.Della V.P., Kuhn I., Hotza D. Rice husk ash as an alternate source for active silica production. Mater Lett. 2002;57:818–821. [Google Scholar]
- 10.de Sousa A.M., Visconte L., Mansur C., Furtado C. Silica sol obtained from rice husk ash. Chem Technol. 2009;3:321–326. [Google Scholar]
- 11.Tsai M.S. The study of formation colloidal silica via sodium silicate. Mater Sci Eng B. 2004;106:52–55. [Google Scholar]
- 12.Lee G.J., Cutler I.B. Formation of silicon carbide from rice hulls. Am Ceram Soc Bull. 1975;54:195–198. [Google Scholar]
- 13.Sujirote K., Leangsuwan P. Silicon carbide formation from pre-treated rice husks. J Mater Sci. 2003;38:4739–4744. [Google Scholar]
- 14.Krishnarao R.V., Godkhindi M.M. Distribution of silica in rice husk and its effect on the formation of SiC. Ceram Int. 1992;18:243–249. [Google Scholar]
- 15.Krishnarao R.V., Godkhindi M.M., Chakraborty M., Mukunda P.G. Direct pyrolysis of raw rice husks for maximization of SiC whiskers formation. J Am Ceram Soc. 1991;74:2869–2875. [Google Scholar]
- 16.Krishnarao R.V., Godkhindi M.M. Maximization of SiC whiskers yield during pyrolysis of burnt rice husks. J Mater Sci. 1992;27:1227–1230. [Google Scholar]
- 17.Krishnarao R.V. Effect of cobalt chloride treatment on the formation of SiC from burnt rice husks. J Eur Ceram Soc. 1993;12:395–401. [Google Scholar]
- 18.Krishnarao R.V. Formation of SiC whiskers from rice husk silica and carbon black mixture; effect of pre-heat treatment. J Mater Sci Lett. 1993;12:1268–1271. [Google Scholar]
- 19.Krishnarao R.V., Godkhindi M.M. Effect of Si3N4 additions on formation of whiskers from rice husks. Ceram Int. 1992;18:185–191. [Google Scholar]
- 20.Ali M. Lambert Academic Publishing; Germany: 2012. Synthesis of silicon carbide and silicon nitride using biomass husks. [Google Scholar]
- 21.Rahman I.A., Riley F.R. Control of morphology in Si3N4 powder prepared from rice husks. J Eur Ceram Soc. 1989;5:11–22. [Google Scholar]
- 22.Rahman I.A. Formation of different Si3N4 phases in presence of V2O5 during carbothermal reduction of untreated and acid-treated rice husks. Ceram Int. 1998;24:293–297. [Google Scholar]
- 23.Sarangi M. Effect of iron catalyst and process parameters on Si-based ceramic materials synthesised from rice husks. Silicon. 2009;1:103–109. [Google Scholar]
- 24.Basu P.K., King C.J., Hynn S. Manufacturing of silicon tetrachloride from rice hulls. AIChE J. 1973;193:439–445. [Google Scholar]
- 25.Chen J.-M., Chang F.-W. Chlorination kinetics of rice husk. Ind Eng Chem Res. 1991;30:2241–2247. [Google Scholar]
- 26.Seo E.S.M., Andreoli M., Chiba R. Silicon tetrachloride production by chlorination method using rice husk as raw material. J Mater Proc Technol. 2003;141:351–356. [Google Scholar]
- 27.Kratel G, Loskot S. Process for the preparation of silicon tetrachloride. USA Invent. Patent No. 1986;4:604-272.
- 28.Bajpai P.K., Rao M.S., Gokhale K.V.G.K. Synthesis of mordenite type zeolite using silica from rice husk ash. Ind Chem Res Dev. 1981;20:721–726. [Google Scholar]
- 29.Wang H.P., Lin K.S., Huang Y.J., Li M.C., Tsaur L.K. Synthesis of zeolite ZSM-48 from rice husk ash. J Hazard Mater. 1998;58:147–152. [Google Scholar]
- 30.Ramli Z., Bahurji H. Synthesis of ZSM-5 type zeolite using crystalline silica of rice husk ash. Malaysian J Chem. 2003;5:45–48. [Google Scholar]
- 31.Ramli Z., Listiorini E., Hamdan H. Optimization and reactivity study of silica in the synthesis of zeolite from rice husk. J Tech UTM. 1996;25:27–35. [Google Scholar]
- 32.Ajay k., Kalyani M., Devendra K., Om P. Properties and industrial applications of rice husk: a review. Int J Emerg Technol Adv Eng. 2012;2:86–90. [Google Scholar]
- 33.Mansary K.G., Ghaly A.G. Thermogravimetric analysis of rice husks in an air atmosphere. Energy Sources. 1998;20:653–663. [Google Scholar]
- 34.Mansary K.G., Ghaly A.G. Thermal degradation of rice husks in an oxygen atmosphere. Energy Sour. 1999;21:453–466. [Google Scholar]
- 35.Mansary K.G., Ghaly A.G. Kinetics of thermal degradation of rice husks in nitrogen atmosphere. Energy Sour. 1999;21:773–784. [Google Scholar]
- 36.Mansary K.G., Ghaly A.G. Determination of kinetic parameters of rice husks in oxygen using thermogravimetric analysis. Biomass Bioenergy. 1999;17:19–31. [Google Scholar]
- 37.Mansary K.G., Ghaly A.G. Thermal degradation of rice husks in nitrogen atmosphere. Bioresour Technol. 1998;65:13–20. [Google Scholar]
- 38.Flynn J.H., Wall L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–328. [Google Scholar]
- 39.Chakraverty A., Mishra P., Banerjee H.D. Investigation of combustion of acid-leached rice husk for production of pure amorphous white silica. J Mater Sci. 1988;23:21–24. [Google Scholar]
- 40.Markovska I.G., Bogdanov B., Nedelchev N.M., Gurova K.M., Zagorcheva M.H., Lyubnchev L.A. Study on thermochemical and kinetic characteristics of alkali treated rice husk. J Chin Chem Soc. 2010;57:411–416. [Google Scholar]
- 41.Ndazi B.S., Nyahumwa C., Tesha J. Chemical and thermal stability of rice husks against alkali treatments. BioResources. 2007;3:1267–1277. [Google Scholar]
- 42.Sharma A., Rao T.R. Kinetics of pyrolysis of rice husk. Bioresour Technol. 1999;67:53–59. [Google Scholar]
- 43.Hu S., Xiang J., Sun L., Xu M., Qiu J., Fu P. Characterisation of char from rapid pyrolysis of rice husk. Fuel Process Technol. 2008;89:1096–1105. [Google Scholar]
- 44.Markovska I.G., Lyubchev L.A. A study on the thermal destruction of rice husk in air and nitrogen atmosphere. J Therm Anal Calorim. 2007;89:809–814. [Google Scholar]