Abstract
Purpose
Patients with advanced stages of MCL have a poor prognosis after standard therapies. MCL cells in those patients often spread into tissues other than lymph nodes, such as the bone marrow. Apart from directed migration and homing, there is little understanding of the function of the CXCR4/SDF1 signaling axis in MCL. In this report, we aim to understand mechanisms of MCL cell survival in the bone marrow.
Experimental Design
For comprehensive analyses of MCL interactions with bone marrow stromal cells we have generated gene knock out cells using CRISPR-CAS9 system and gene knock down cells to reveal novel roles of the CXCR4/SDF-1 signaling.
Results
CXCR4 silencing in MCL cells led to a significant reduction in proliferation, cell adhesion to bone marrow stromal cells and colony formation in PHA-LCM methylcellulose medium, which were reversed upon the addition of SDF-1 neutralizing antibodies. In addition, tracking MCL cell engraftment in vivo revealed that quiescent MCL cells are significantly reduced in the bone marrow upon CXCR4 silencing, indicating that CXCR4/SDF-1 signaling is required for the survival and maintenance of the quiescent MCL cells. Further analysis revealed novel mechanisms of ROS induced CXCR4/SDF-1 signaling that stimulate autophagy formation in MCL cells for their survival.
Conclusions
Our data, for the first time, revealed new roles of the CXCR/SDF-1 signaling axis on autophagy formation in MCL, which further promoted their survival within the bone marrow microenvironment. Targeting the CXCR4/SDF-1/autophagy signaling axis may contribute to an enhanced efficacy of current therapies.
Keywords: Mantle cell lymphoma, Autophagy, Bone marrow microenvironment, CXCR4, SDF-1
INTRODUCTION
Mantle Cell Lymphomas (MCL), a rare but particularly deadly sub-type of Non-Hodgkin’s Lymphoma (NHL), are refractory to conventional therapies and display cellular heterogeneity and genomic instability (1-3). The major genetic alteration in MCLs that distinguish them from low-grade B cell lymphomas is the t(11;14)(q13;q32) translocation, leading to increased levels of cyclin D1 (CCND1) gene expression (2). Although this translocation is a genetic hallmark of most MCLs, CCND1 overexpression is not sufficient to induce MCL. For example, transgenic mice overexpressing CCND1 in B cells do not show increased lymphoma incidence (4, 5). Additionally, the t(11;14)(q13;q32) translocation exists in blood cells in approximately 2% of healthy individuals without evidence of disease (6), and some confirmed MCLs lack any translocation affecting the 11q13 locus (2, 7). Collectively, these results suggest that other genetic or epigenetic events, possibly acting cooperatively with CCND1 overexpression, are required for the development of MCL.
Although there have been improvements in overall survival (OS), the prognosis of MCL is still one of the worst among NHL (8). Relapsed and high-grade MCL patients often demonstrate the presence of MCL cells in other tissues, such as the bone marrow and lymphatic tissues, which are critical for disease progression (2, 3).
Chemokine stromal cell-derived factor-1 (SDF-1/CXCL12) is expressed by stromal marrow cells. Its receptor, CXCR4, plays critical roles in targeting hematopoietic stem cells (HSCs) within the marrow microenvironment (9), and the CXCR4 inhibitor AMD3100 (Plerixafor) has been shown to induce significant HSC mobilization into the peripheral blood (10). The SDF-1/CXCR4 signaling axis has been reported to play an important role in proliferation, metastasis and angiogenesis in many cancers, such as breast (11), glioblastoma (12), melanoma (13), pancreatic (14) and lung (15, 16).
Even though the presence of MCL cells in bone marrow is a negative prognosis factor for MCL patients, very limited research has been reported regarding biological mechanisms of MCL cell survival in the bone marrow (17). In this study, we show, for the first time, that the CXCR4/SDF-1 signaling axis contributes to MCL cell survival within the bone marrow compartment via autophagy. Silencing CXCR4 in MCL cells led to decreased proliferation and colony formation, indicating that the CXCR4/SDF-1 signaling axis can contribute stem-like properties in MCL similar to its function in HSCs. MCL colony formation was markedly increased upon co-culturing with human bone marrow stromal cells, HS27a or SDF-1. Moreover, the increase of cell survival under stressed conditions involved autophagy, an evolutionarily conserved process that targets cellular materials to the lysosome for degradation. Beclin1 silencing in MCL cells led to reduced cell survival and bone marrow targeting without affecting CXCR4 cell surface expression. In summary, our study shows novel mechanisms of MCL cell survival in the bone marrow compartment and is the first report on the regulation of the CXCR4/SDF-1 signaling axis in autophagy in any cancer.
Understanding the molecular mechanisms that confer growth and dispersal to MCL cells will provide possible avenues for targeting these signaling pathways in MCL.
MATERIALS AND METHODS
Cell lines
The human mantle cell lymphoma cell lines SP-53, Jeko, Mino and Z138 were obtained from the American Type Culture Collection (Manassas, VA). HS27a bone marrow stromal cells (BMSCs) were a kind gift from Dr. B. Torok-Storb (Fred Hutchinson Cancer Research Center, Seattle, WA). Authentification of cell lines was done in 2014 using DNA fingerprinting.
Reagents and antibodies
Anti-CXCR4 (12G5) antibodies were purchased from BD Bioscience. Anti-LC3 (1:500) antibodies was purchased from Novus Biologicals (Littleton, CO). SDF-1 was purchased from Pepro Tech (Rocky Hill, NJ), and neutralizing antibodies against SDF-1 (MAB310) were purchased from R&D system (Minneapolis, MN).
Lentivirus generation and transfection
293FT cells were transfected with a target plasmid containing a GFP construct and shRNA specific for human CXCR4 (V3LHS_640647, Open Biosystem, Lafayette, CO). Vectors lacking the shRNA but containing GFP served as controls. We followed standard protocols to generate lentivirus. Briefly, 293FT cells were transfected with both pMD2.G (envelope plasmid) and psPAX2 (packaging plasmid) using calcium phosphate precipitation, and the lentiviruses were collected 48 h post transfection. Different lymphoma cell lines were infected by lentiviruses encoding the CXCR4 shRNA and were sorted 96 h post infection for GFP positive cells. Following sorting, cell lines were selected using puromycin (4 μg/mL) or blasticidin (2 μg/mL). Cells were selected for 14 days to generate stable cell lines.
Cell adhesion assay
HS27a BMSCs were seeded at a density of 5 × 104 cells/well in a 24-well plate and allowed to form a monolayer over 48-72 h. MCL cell lines were stained with the membrane dye PKH26 according to the manufacturer’s protocol (Sigma, St. Louis, MO). MCL cells (3 × 105) suspended in 200 μL of complete RPMI1640 were then plated onto the pre-established monolayer of HS27a cells and allowed to adhere for 1 h at 37°C. Wells were then washed twice with 1 × HBSS, and PKH26 dye intensity was measured using an Infinite®M1000 (TECAN) fluorescent plate reader. Wells containing MCL cells with no wash step served as a 100% adhesion control, while wells containing just the monolayer of HS5 cells served as a 0% adhesion control.
Motility assay
MCL cells were stained with the membrane dye PKH26. Cells (3 × 104) were suspended in 100 μL of complete RPMI1640 were then plated onto empty 96-well plates or onto plates containing a pre-established monolayer of HS27a cells. The cells were allowed to rest for 1 h at 37°C before having their motions recorded using a time-lapse microscopy. Time-lapse images were acquired with an Andor IXon3 885 EMCCD camera (Andor, South Windsor, CT) on an Olympus IX-81 (Olympus, Center valley, PA) microscope fitted with a microscope enclosure (Precision Plastics) maintained at 37°C with 5% CO2.
Cell cytotoxicity assays
MCL cells (2 × 104 in 100 μL) were plated onto 96-well plates and supplemented with HS27a conditioned media or cultured with established HS27a BMSCs. Different chemical compounds were added at the indicated concentrations, and cell viability was tested using CellTiterBlue® (Promega, Madison, WI) at 4 h according to the manufacturer’s recommendations.
MethoCult™ colony assay
MCL cells (5000 cells) were suspended in 1 mL of complete MethoCult™ medium and plated onto 35-mm petri dishes. Colonies were maintained at 37°C with 5% CO2 and 95% humidity for 7 days. Colonies were counted and scored at day 7, and pictures were taken using an Eclipse TE200-E microscope (Nikon). Only colonies consisting of 50 or more cells were considered for analysis.
PKH staining and calculation of recovery rates
MCL cells (5 × 106) were stained with PKH26 (Sigma-Aldrich, final concentration of 2 × 10−6 M) for 5 min. All of the procedures followed a manufacturer’s protocol. Stained cells were examined for PKH expression using FACS analysis to ensure that more than 99% of the cells were PKH positive. These cells (106) were injected into irradiated (225 Gy) NOD/SCID mice, which were subsequently sacrificed after 48 h. The recovery rates of the PKH-positive cells were calculated using the following formula: % recovery = (# of PKH bright cells recovered) / (# of PKH bright cells injected).
RESULTS
The CXCR4/SDF1 signaling axis supports MCL survival under a stressed condition
Although SDF-1 is a major chemokine required for early B cell development and bone marrow homing (18), only one study has investigated the role of CXCR4, which showed a reduction of MCL migration (19). To delineate CXCR4’s biological function on MCL cells, we generated stable MCL cell lines in which their CXCR4 levels were silenced using a lentivirus encoding a CXCR4 shRNA or a control shRNA. All stable cell lines were selected for GFP expression and antibiotic resistance (Materials and Methods). The levels of CXCR4 expression varied among different MCL cell lines, but most cells maintained a relatively high expression of CXCR4 on the surface (Supplemental Figure 1). FACS analyses of GFP-positive lentivirus infected MCL cells showed a 74-94% reduction of CXCR4 expression in GFP-positive MCL cells compared to GFP-positive control MCL cells (Supplemental Figure 1). As expected, these CXCR4 silenced cells showed significantly decreased migration toward different concentrations of SDF-1 in transwell assays compared to the migration observed by the control-shRNA cells (Supplemental Figure 2). To investigate the roles of CXCR4/SDF-1 signaling in MCL cells in the bone marrow microenvironment, we utilized HS27a human bone marrow stromal cells (BMSCs), which were reported to produce large amounts of SDF-1 (20). Similar to the results observed in the migration toward recombinant SDF-1, CXCR4-silenced MCL cells showed a significant reduction in their migration toward the medium harvested from the HS27a culture (Supplemental Figure 3).
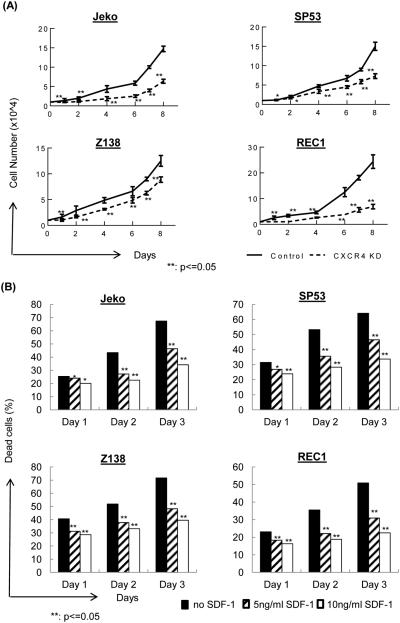
These GFP-positive CXCR4-shRNA MCL cells were then analyzed for cell growth in vitro. CXCR4 silencing led to similar growth rates as the MCL cell controls at day 1-3; however, after extended culture periods, GFP-positive CXCR4-shRNA MCL cells showed largely suppressed growth compared to that of the control-shRNA MCL cells (Figure 1A). We then incubated MCL cells in low serum (2%) to evaluate the roles of the CXCR4/SDF-1 signaling axis in a stressed condition. Compared to MCL cells whose survival was markedly reduced under low serum conditions, providing SDF-1 during starvation led to a reduction of cell death, which was evaluated by 7AAD/annexin V (Figure 1B). This result suggests that the CXCR4/SDF-1 signaling axis can provide protective signaling in MCL cells during a starvation condition. The addition of recombinant SDF-1α during culture protected the cells from apoptosis, and these protective effects were reversed when the cells were incubated with neutralizing SDF-1α antibodies (Supplemental Figure 4).
Figure 1.
(A) CXCR4 silencing in MCL cells leads to reduced cell growth. CXCR4 was silenced in four different MCL cell lines using a CXCR4 shRNA-encoding lentivirus or control lentivirus. After infection, cells were sorted for GFP and were selected using antibiotics. These stable cell lines (1×104) were incubated in 24-well plates for 10 days to evaluate their growth. Cells were counted in triplicates every day.
(B) SDF-1 protects MCL cells from starvation-induced cell death. MCL cells (1×106) were cultured in low serum for three days, and SDF-1 (5 ng/ml or 10 ng/ml) was added to the culture daily. The cells were evaluated using 7-AAD/Annexin V staining followed by FACS analyses.
The CXCR4/SDF-1 signaling axis contributes to the stem-like colony forming activities in PHA-LCM medium
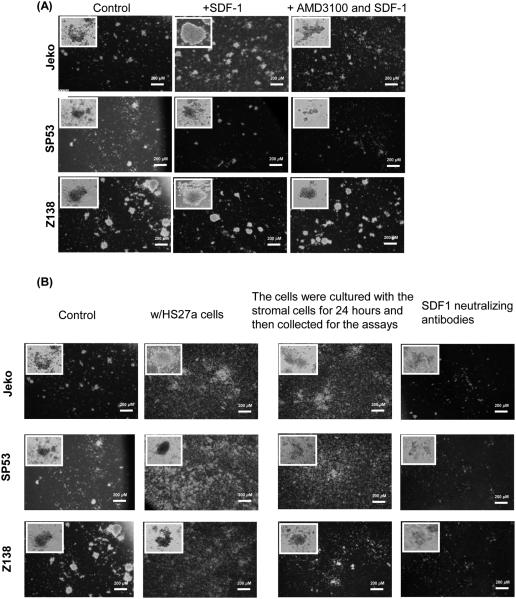
SDF-1 is secreted by stromal and endothelial cells in many organs (21, 22), where it recruits CXCR4-expressing cells. We tested the effects of the CXCR4/SDF-1 signaling axis on colony forming activities using PHA-LCM (Phytohemagglutinin human leukocyte conditioned medium) conditioned medium; PHA-LCM has been used to characterize stem-like cells in multiple myeloma (23). SDF-1 is also a chemokine critical for maintaining HSCs in adult bone marrow (24). The addition of SDF-1 greatly increased the colony forming ability of Jeko, SP53 and Z138 MCL cells in PHA-LCM medium (Figure 2A, Supplemental Table 1). The increase of colony forming activity in MCL cells was due to the presence of exogenous SDF-1 because the addition of AMD3100 (Plerixafor, CXCR4 antagonist) with SDF-1 reversed the effects of SDF-1 (Figure 2A, Supplemental Table 1). To test the importance of the CXCR4/SDF-1 signaling axis on the colony forming ability of MCL cells in a more physiological condition, we co-cultured HS27a BMSCs with MCL cells in PHA-LCM medium. Co-culturing MCL cells with HS27a BMSCs and stimulating the MCL cells with HS27a prior to plating effectively increased the colony forming activities in PHA-LCM medium (Figure 2B, Supplemental Table 2). The increase in colony forming activity was due to the SDF-1 secreted by the stromal cells. Blocking SDF-1 with neutralizing antibodies largely reduced the colony forming ability of the MCL cells (Figure 2B, Supplemental Table 2). Because the colonies that formed in the PHA-LCM medium contained an identical light chain arrangement to that of the MCL cells, it is likely that the colonies originated from MCL cells (Supplemental Figure 5).
Figure 2.
(A) Jeko, SP53 and Z138 MCL cells (5×103) were cultured in PHA-LCM (phytohemagglutinin human leukocyte conditioned medium) methylcellulose medium. To test the role of the CXCR4/SDF-1 signaling axis in colony formation in PHA-LCM medium, SDF-1 (200 ng/ml) or AMD3100 (40 μM) or AMD3100 with SDF-1 were added during culture. Cells were counted as a colony if they had more than 40 cells. Colony numbers were combined from triplicate plates.
(B) MCL cells were cultured in PHA-LCM medium with human bone marrow stromal cells, HS27a, or SDF-1 neutralizing antibodies (100 mg/ml). In some experiments, MCL cells were also cultured using stromal cells for 24 hours and collected for the colony forming assays.
The CXCR4-SDF1 signaling axis is critical for the survival of quiescent MCL cells in bone marrow and for MCL cell adhesion to bone marrow stromal cells
The bone marrow microenvironment can be divided into two distinct niches: osteoblastic and vascular. Our recent study revealed that quiescent multiple myeloma (MM) cells prefer to reside within the osteoblastic niche (25). Therefore, we utilized a similar approach and labeled GFP-positive CXCR4 control and silenced cells with PKH26 dyes to comprehensively study the role of CXCR4/SDF-1 signaling.
To quantify the effects of CXCR4 silencing in vivo on MCL cell homing and to investigate whether CXCR4 signaling influences quiescent MCL cell engraftment to the particular niches within the bone marrow, we transplanted PKH-positive GFP-positive MCL cells into NOD/SCID mice. We allowed the PKH-labeled GFP-positive MCL cells to undergo the cell cycle within NOD/SCID mice for 48 hours before we tracked the PKH-positive cells in different niches (25). After cycling, the remaining PKH-stained cells can be used to observe quiescent cells within a proliferating population (26, 27). This method has been used to study HSCs (27) as well as stem cell-like cells in breast cancer and multiple myeloma (25, 28). Given that the CXCR4/SDF-1 signaling axis plays important roles in colony formation in vitro (Figure 2) and in maintaining HSCs within the bone marrow niche (9), it will be important to analyze the role of this signaling axis in maintaining quiescent MCL populations in the bone marrow microenvironment.
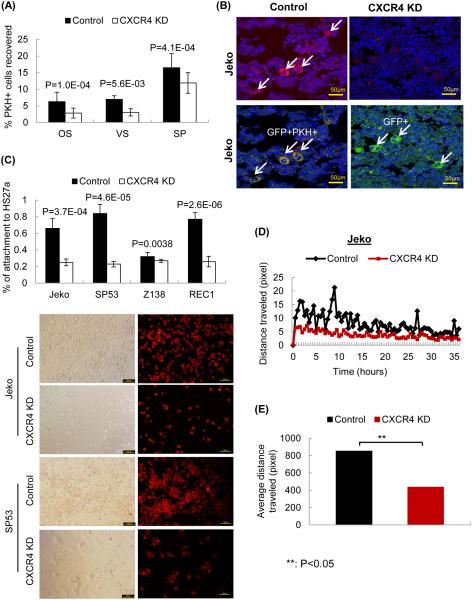
At 48 hours post-transplant, the recipient mice were sacrificed for the analysis of PKH-positive cell recovery. Similar to the HSCs and MM cells reported previously (25, 27), the highest amounts of PKH-positive cells were recovered after 48 h of in vivo cycling (data not shown). Therefore, we performed all of the following analyses 48 h post-transplantation. The numbers of PKH26-positive cells in each niche were analyzed using FACS. After averaging a total of 17 mice, 2.8% of the PKH26-positive CXCR4-shRNA Jeko cells were recovered from the osteoblastic niche, 3% from the vascular niche, and 12% from the spleen compared to 6.29%, 6.96%, and 16.5% of control cells, respectively (Supplemental Table 3 and Figure 3A). After CXCR4 silencing, PKH26-positive MCL cells recovered from the osteoblastic and the vascular niches were reduced by 55% and 56%, respectively, whereas the recovery from spleens was reduced by 26%.
Figure 3.
(A) Decreased quiescent PKH-positive cell recovery in vivo upon CXCR4 silencing. GFP-positive CXCR4 shRNA MCL cells (CXCR4 KD) or control shRNA cells (control, 5×105) were labeled using the fluorescent dye PKH26 and were injected into NOD/SCID mice to evaluate engraftment. Mice were sacrificed after 48 hours, and the cells were isolated from the osteoblastic niche, vascular niche of the bone marrow and spleens. PKH-positive cell recovery was calculated as described in the Methods.
(B) Immunohistochemical analyses confirmed the FACS data. Confocal microscopy was performed using different sections of paraffin embedded bones. The bones were isolated from xenograft NOD/SCID mice. PKH26-positive cells are shown in red, GFP-positive MCL cells are shown in green, and the nucleus is shown in blue. This confocal staining is in accordance with the observations from the FACS analysis, i.e., the PKH-positive engraftment decreased upon CXCR4 silencing.
(C) CXCR4-silenced MCL cells significantly decreased attachment to HS27a bone marrow stromal cells. CXCR4-shRNA or control-shRNA MCL cells (5×104) were labeled with PKH26 and incubated for 1 hour with HS27a stromal cells. PKH26 was used for visualization, and its intensity did not decrease within the 1-hour assay period. The percentages of MCL cell attachment to HS27a were calculated.
(D & E) CXCR4 silencing decreases the motility of MCL cells. We quantitatively measured the distances the CXCR4-silenced or control cells travelled over 2 days using a time-lapse microscopy. Distances (mm) traveled by CXCR4-silenced Jeko cells, which were an average of results from 15 different wells, were significantly decreased compared to the control cells.
Unlike the MM cells, which showed increased homing to the bone marrow compared to the spleen, higher numbers of PKH-positive MCL cells migrated to the spleen than to the bone marrow. However, after CXCR4 silencing, quiescent PKH-positive cell migration to the bone marrow was less than PKH-positive cell migration to the spleen (Supplemental Table 3), indicating that the CXCR4/SDF-1 signaling axis plays an important role in the survival of quiescent MCL cells in the bone marrow. In the bone marrow, SDF-1 is predominantly expressed by endothelial cells as well as endosteal bone lining osteoblasts (29).Therefore, quiescent MCL cell recovery between the osteoblastic and the vascular niches was similar upon CXCR4 silencing. Immunohistochemical analyses of xenograft bone marrow confirmed the FACS data. Engraftment of PKH26-positive (red) cells, which label quiescent MM cells, was significantly decreased upon CXCR4 silencing (Figure 3B). Because the assays were performed within 48 hours, the engraftment levels of GFP-positive (green) cells were similar among the injected mice.
To further analyze the importance of the CXCR4/SDF-1 signaling axis in the interaction between MCL cells and BMSCs in vitro, we performed cell adhesion assays using stromal cells. GFP-positive CXCR4-shRNA MCL cells were labeled with red fluorescent dyes to easily track the cells. Compared to control-shRNA cells, Jeko, SP53 and Rec CXCR4-silenced cells demonstrated a marked decrease in cell adhesion to HS27a BMSCs (Figure 3C).
We then analyzed the movement of the CXCR4-silenced or control cells on the stromal cells by quantitatively measuring the distance they traveled using a time-lapse microscope. Both cell types were incubated on HS27a BMSCs, and the distances (μM) that they traveled were measured every 30 min for 3 days. Based on an average of the results from 8 different wells, CXCR4-silenced Jeko (Figures 3D & 3E), SP53 and Rec cells (Supplemental Figure 6) showed a significantly reduced travel distance compared to that of the control cells (. Collectively, these results demonstrate that CXCR4/SDF-1 signaling is a critical component for MCL cell interaction with BMSCs and for the survival of quiescent MCL cells within the bone marrow compartment.
Bortezomib-resistant MCL cells upregulate expression of CXCR4 which is mediated by ROS
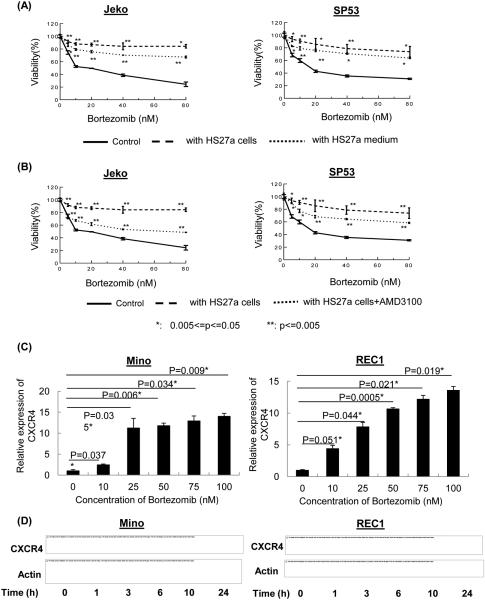
Because stromal-secreted SDF-1 stimulated MCL cell proliferation, adhesion and colony formation, we analyzed whether stromal cells can protect MCL cells from chemotherapeutic-derived cytotoxicity. We chose to test bortezomib because it has been used to treat relapsed MCL patients (30) and there are commercially available bortezomib-resistant and bortezomib-susceptible MCL cell lines. As expected, there were significant differences in bortezomib susceptibility between the susceptible and resistant MCL lines (Supplemental Figure 7). We incubated bortezomib susceptible MCL cell lines as well as patient cells with HS27a BMSCs and analyzed cytotoxic responses after bortezomib treatment. Co-culturing MCL cells with HS27a stromal cells or media from HS27a cells showed protective effects against bortezomib, and this protective effect was diminished after the addition of AMD3100 (Figures 4A & B, Supplemental Figures 8A and B), indicting SDF-1/CXCR4 signaling is involved in stromal cell mediated bortezomib resistance in susceptible cells.
Figure 4.
(A) HS27a stromal cells as well as HS27a media protect MCL cells from chemotherapeutic drug cytotoxicity. Jeko and SP53 MCL cells (2×105) were cultured with HS27a stromal cells or 1 ml of 1:1 diluted HS27a medium. Cell viability was determined by the CellTiter-Blue® fluorometric assay (Promega) and was indicated as a ratio compared to cell viability without treatment. Bortezomib was serially diluted (0-80 nM) as indicated. The results represent the mean ± standard deviation of triplicates. *, P<0.05; **, P<0.005. P values were calculated using Student’s t-test.
(B) AMD3100 treatment abolishes the protective effects of the stromal cells against chemotherapeutic drugs. A CXCR4 antagonist, AMD3100 (40 μM), was incubated with MCL cells for 24 hours, and cell viability was determined by CellTiter-Blue. The results represent the mean ± standard deviation of triplicates. *, P<0.05, **, P<0.005; P values were calculated using Student’s t-test.
(C-D) Bortezomib treatment induces CXCR4 expression in MCL cells. Bortezomib-resistant Mino and REC1 cells (106, 6-well plate) were treated with different doses of bortezomib (0-100 nM for 24 hours) (C) or with a constant dose (50 nM) of bortezomib for different time intervals (D). CXCR4 expression was analyzed by quantitative RT-PCR (C) or PCR (D). GAPDH (qRT-PCR) and b-actin (PCR) were used as internal controls. The results show that bortezomib induces a dose- and time-dependent expression of CXCR4 in bortezomib-resistant MCL cell lines. Bars represent the average of triplicates with standard deviation. All values were statistically significant compared to untreated samples.
In order to further investigate cell intrinsic survival mechanisms in bortezomib resistant MCL cells (Mino and REC1) and roles of SDF-1/CXCR4 axis in that process, we explored the effects of bortezomib on CXCR4 expression in MCL cells by real time PCR. After treatment with bortezomib (0-100 nM) for 24 hours, a dose-dependent increase in CXCR4 mRNA was observed in Rec1 and Mino bortezomib-resistant MCL cell lines (Figure 4C). In a time-course assay (0-24 h), we also observed, by PCR, increases in CXCR4 gene expression (Figure 4D) and protein production by FACS analyses (Supplemental Figure 9) after bortezomib treatment. Since several studies reported ROS effects after bortezomib treatment (31, 32), we examined roles for ROS in bortezomib-induced CXCR4. Bortezomib resistant MCL cells were treated with N-acetyl-L-cysteine (NAC) one hour prior to bortezomib treatment. The FACS data evaluating CXCR4 cell surface expression showed that effects of bortezomib on CXCR4 expression are abolished in NAC-treated cells (Supplemental Figure 10). We then tested the effects of stromal cells on cytotoxicity of IMBRUVICA (Ibrutinib), an inhibitor of Bruton’s tyrosine kinase. IC50 of Ibrutinib between MCL cell lines displayed some differences as expected (Supplemental Figure 11). Co-culturing MCL cell lines as well as patient cells with HS27a stromal cells or media from HS27a cells showed some protective effects against Ibrutinib (Supplemental Figure 12). However, treating MCL cells with Ibrutinib did not increase ROS, indicating ROS is not a part of mechanisms of Ibrutinib cytotoxicity (Supplemental Figure 13). Ibrutinib treatment also did not increase CXCR4 expression by FACS (Supplemental Figures 14 and 15). It would be interesting to investigate in the future the mechanisms of SDF-1 and Ibrutinib-related MCL resistance. Collectively, our data support that CXCR4 expression is increased in bortezomib resistant MCL cells in a time- and dose-dependent manner via ROS.
Drug resistant MCL cells upregulate autophagy for survival
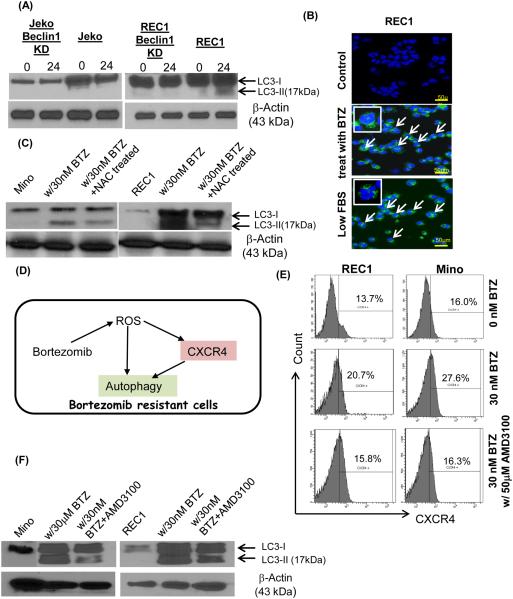
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved catabolic pathway in which macromolecules and organelles are sequestered into autophagosomes and subsequently fused with the lysosome, where the content is digested and recycled (33, 34). Autophagy was reported to play a pro-survival role in MCL cells that are resistant to everolimus (RAD001), an mTOR inhibitor (35). Since bortezomib treatment and ER stress were reported to trigger autophagy production, we investigated whether autophagy can be utilized in MCL cells as an intrinsic way to survive after bortezomib treatment. Bortezomib (30 nM) was added for varying times (0 and 24 hours) to Rec1 (bortezomib-resistant) and Jeko (bortezomib-sensitive) cells. In Rec1 cells, bortezomib treatment led to the processing of LC3B-I to LC3B-II, indicating increased autophagic activity (Figure 5A). Chloroquine treatment increased LC3B-II band relative to non-treatment samples, which further confirmed increased autophagy (Supplemental Figure 16). However, bortezomib did not induce robust autophagy in bortezomib-susceptible Jeko cells (Figure 5A). We then generated beclin 1 knock down cells using recombinant lentivirus. GFP+ beclin 1 knock down cells demonstrated severely reduced beclin 1 expression (Supplemental Figure 17). The induction of autophagy was diminished upon expressing beclin1 shRNA in Rec1 cells (Figure 5A). When the GFP-LC3 plasmid is overexpressed in cells, the expressed protein is spread diffusely throughout the cytoplasm. The induction of autophagy by nutrient starvation causes GFP-LC3 to be integrated into autophagosomes and to be displayed as punctate structures (36). Distinct GFP-LC3 punctate structures were observed in bortezomib treated REC1 cells with low serum treatment as a positive control (Figure 5B). We also treated the cells with NAC to test whether reduction of ROS affects autophagy formation. Inhibiting ROS using NAC dramatically reduced autophagy formation in drug resistant MCL cells (Figure 5C).
Figure 5.
(A) Bortezomib increased autophagy in drug resistant MCL cells but not in susceptible cells. Bortezomib (30 nM) was added to the MCL cells for 24 hours, and autophagy induction was analyzed based on the LC3B-I to LC3B-II conversion as measured by immunoblots. Cells were harvested at 0 and 24 hours. Only live cells were used for analyses. Beclin knock down REC1 cells showed significant decreased LC3B-I to LC3B-II conversion.
(B) The GFP-linked LC3 (GFP-LC3) was transiently infected into MCL cells to evaluate autophagy induction. When autophagy is occurring, LC3 is concentrated in autophagosomes and shows punctate green fluorescence. Compared to the controls, MCL cells incubated in low serum (positive control) or with bortezomib (30 nM) showed distinct punctate structures, indicating autophagy induction.
(C) Autophagy formation was reduced in drug resistant MCL cells after NAC treatment with bortezomib. Mino and REC1 cells were treated with 30 nM bortezomib for 24 hours with or without pretreatment of NAC (100 μM).
(D) A model diagram depicting observations made in the study. Chemotherapeutic drugs that produce ROS such as bortezomib can induce upregulation of CXCR4, which in turns, stimulate autophagy formation. Upregulated autophagy formation further supports the drug resistant MCL cell survival.
(E) Upregulated CXCR4 expression after bortezomib treatment (30 nM) was reduced to the levels without bortezomib upon AMD3100 (50 μM) treatment.
(F) Autophagy formation was reduced in Mino and REC1 MCL cells with bortezomib (30 nM) and AMD3100 treatment (50 μM) compared to the cells treated with only bortezomib (30 nM).
CXCR4 inhibition affects autophagy formation in MCL after bortezomib treatment
Since bortezomib treatment induced CXCR4 upregulation and autophagy via ROS and (Figure 5D), we further investigated a primary driver of survival mechanism in bortezomib resistant cells. We inhibited CXCR4 upregulation after bortezomib using AMD3100. AMD3100 treatment effectively reduced CXCR4 levels to the levels before bortezomib treatment (Figure 5E). Reduction of CXCR4 by AMD3100 reduced autophagy formation in Mino and REC after bortezomib treatment (Figure 5F). Collectively, our data indicates that drug resistant MCL cells utilize not only ROS generated after drug treatment but also utilize increased CXCR4 signaling to upregulate autophagy to benefit their survival.
CXCR4 is a key signal in autophagy induction in bortezomib-sensitive MCL cells
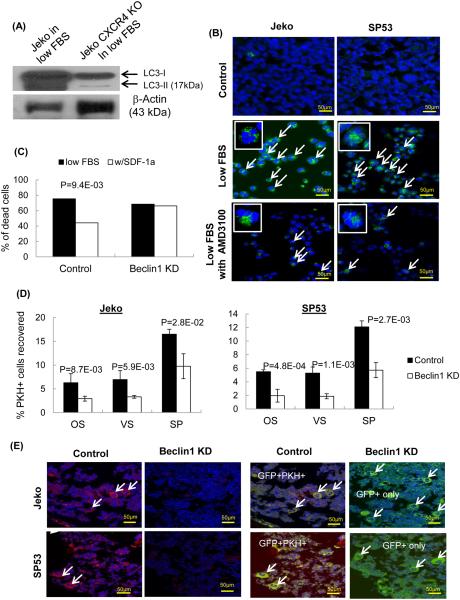
Bortezomib treatment (30 nM) of Jeko did not increase autophagy formation (Figure 5A). In order to further dissect interplays between autophagy and CXCR4 in bortezomib susceptible cells, we used CXCR4 knock out Jeko cells generated by CRISPR-CAS9. Since 30 nM of bortezomib treatment will result in killing most of bortezomib susceptible cells, we cultured these cells in low serum to test whether deleting CXCR4 signaling affects levels of autophagy. When CXCR4 KO Jeko or control cells were incubated in low serum, control cells showed robust autophagy formation, however CXCR4 KO Jeko cells showed drastically compromised autophagy formation (Figure 6A). We also treated these MCL cells with AMD3100, which showed similar reduction of autophagy formation in low serum condition in immunoblot analyses (Supplemental Figure 18) as well as confocal analyses (Figure 6B).
Figure 6.
(A) CXCR4 KO cells were generated using CRISPR-CAS9 system. CXCR KO Jeko MCL cells showed dramatically reduced autophagy formation with low serum culture. Immunoblots analyses showed conversion of LC3-I to LC3-II.
(B) Confocal analyses showed distinct punctate green fluorescence in Jeko and SP53 cells. Compared to the low serum controls, MCL cells incubated in low serum with AMD3100 showed reduced punctate structures compared to MCL cells with low serum, indicating reduction of autophagy formation.
(C) Survival of Jeko cells was not rescued in low serum upon SDF-1 addition\ without Beclin1. Beclin1 shRNA Jeko cells (1×105) were incubated in low serum for 3 days with or without SDF-1 (10 ng/ml). Cell death was calculated using 7-AAD/Annexin V.
(D) Beclin1 silencing in MCL cells significantly decreased PKH-positive cell engraftment. GFP-positive Beclin1 silenced Jeko or SP53 cells were then labeled with red fluorescent PKH 26 dye followed by transplantation into NOD/SCID mice. Mice were sacrificed after 48 hours, and the cells were isolated from the osteoblastic vascular niches of the bone marrow and spleens. PKH-positive cell recovery was calculated as described in Methods.
(E) Immunohistochemical analyses confirmed the FACS data. Confocal microscopy was performed using different sections of paraffin embedded bones. The bones were isolated from xenograft NOD/SCID mice. PKH26-positive cells are shown in red, GFP-positive Beclin1-silenced Jeko or SP53 cells are shown in green, and the nucleus is shown in blue.
Beclin1 is a member of the class III PI3kinase complex that is essential for autophagy (37). Since CXCR4 silencing affects autophagy formation in Jeko under low serum condition (Figure 6), we tested whether effects of reduced autophagy in bone marrow homing in vivo using beclin1-silenced Jeko cells. Beclin1-silenced Jeko cells showed similar levels of cell death compared to that of the control cells upon SDF-1 treatment (Figure 6C), suggesting that SDF-1 fails to rescue MCL cells from cell death in the absence of Beclin1. Beclin1 silencing did not affect CXCR4 cell surface expression in MCL cells (Supplemental Figure 19).
We then injected Beclin1-silenced Jeko cells into mice and analyzed the ability of the cells to migrate to the bone marrow microenvironment. Bone marrow is highly maintained in hypoxic condition in vivo (38), any cancer cells migrating to bone marrows must overcome the hostile environment, which induce stress to the cells. Beclin1-silenced Jeko cells showed a significant decrease of cell engraftment in the bone marrow compared to that of the control Jeko cells (Supplemental Table 4, Figure 6D). Immunohistochemical analyses confirmed a significant reduction of PKH-positive cells in the bone marrow (Figure 6E). Collectively, our data supports the idea that the CXCR4/SDF-1 signaling axis plays an important role in the survival of MCL cells in bone marrow via autophagy. Drugs that target this balance may have therapeutic efficacy in patients who have relapsed or have high-grade MCL with bone marrow involvement.
DISCUSSION
Despite the known functions of the CXCR4/SDF-1 signaling axis in the migration of MCL cells (19), it is unknown whether CXCR4/SDF-1 signaling can contribute to the survival of MCL cells; furthermore, the precise mechanisms of this regulation have not been elucidated. CXCR4/CXCL12 signaling induces type III PI3K inhibition which negatively regulates autophagy formation under normal nutritional condition (39). However, we found that under stressed conditions such as chemotherapy, lack of nutrients or low oxygen condition such as within the bone marrow microenvironment, MCL cells use CXCR4 signaling to stimulate autophagy. In drug resistant MCL cells, we observed unwanted effects of chemotherapy treatment, which MCL cells increased CXCR4 expression upon bortezomib treatment, which stimulate autophagy formation for their survival.
HSCs induce protective autophagy to cope with cellular stresses occurring in the bone marrow cavity (40). In MCL, autophagy induction can protect cells from everolimus (RAD001), an mTOR inhibitor, which suggest that targeting autophagy can be an effective therapy to overcome resistance to everolimus (35). Knocking down the ATG4B gene, a critical component for autophagy, impairs the survival of chronic myeloid leukemia (CML) stem/progenitor cells and can sensitize them to imatinib mesylate (IM) treatment, suggesting that ATG4B is as a potential drug target (41).
SDF-1 is secreted by stromal and endothelial cells in many organs (21, 22), where it recruits CXCR4-expressing cells. Downstream signaling by CXCR4 involves the PI3K, ERK and AKT pathways and promotes cell homing. We found that most MCL cells express elevated levels of CXCR4 on the surface and that silencing CXCR4 expression decreased cell proliferation, cell adhesion, migration on bone marrow stromal cells, and colony forming ability. In various cancers, including gliomas (42), breast (43) and ovarian (44), SDF-1 can stimulate the proliferation and/or survival of CXCR4-expressing cancer cells. Additionally, treatment with AMD3465, a second-generation inhibitor of CXCR4/SDF-1, enhances chemotherapy efficacies by inducing the mobilization of AML cells and by abrogating the protective effects of stromal cells (45). In MM, AMD3100 induces the disruption of the interaction of MM cells with bone marrow and the mobilization of MM cells into circulation (46). These results indicate that CXCR4 inhibition can be an effective therapeutic strategy by targeting tumor cell interaction with the bone marrow microenvironment.
While autophagy occurs at a basal level in most tissue types to promote cellular homeostasis, it is upregulated in response to nutrient starvation, hypoxia (47), oxidative stress (48, 49), and chemotherapeutic drugs (50). The bone marrow compartment is maintained as a highly hypoxic environment (38), which could provide a favorable condition for the induction of autophagy. Although there are no reports directly linking hypoxia induction of autophagy in the bone morrow, several studies reported the role of hypoxia in autophagy induction in general (51, 52). Additionally, in gastric cancers, hypoxic conditions upregulate CXCR4 in a hypoxia inducible factor-1α (HIF-1α)-dependent manner (53). Together these results suggest a potential mechanism where a combined CXCR4 and hypoxia signal increases autophagy in the bone marrow; however, a detailed molecular analyses needs to be conducted to explore this potential mechanism.
High-grade MCLs often spread to the bone marrows, which provide a protective niche for the chemotherapeutic drug treatment. Our findings imply that inhibiting CXCR4 signaling or autophagy formation after ROS-inducing chemotherapies such as bortezomib will increase efficacy of the drugs. Our report is the first to show that CXCR4/SDF-1 signaling involves autophagy induction to promote malignant cell survival in any cancer. Understanding the interactions between the bone marrow microenvironment and MCL cells may provide important advances for new therapeutic targets.
Supplementary Material
Key points.
CXCR4/SDF-1 signaling axis regulates MCL cell survival via autophagy
Translational Relevance.
CXCR4 antagonists such as plerixafor (Mozobil/AMD3100) showed some efficacy in chronic lymphocyte leukemia (CLL) by distrupting the interaction between CLL and stromal cells, which lead to recirculate CLL cells towards the bloodstream. However, the therapeutic potential of CXCR4 antagonists are relatively unknown in lymphomas. Using mantle cell lymphoma as a model system, we discovered new roles of the CXCR/SDF-1 signaling axis on autophagy formation in MCL, which further promoted their survival within the bone marrow microenvironment. Our data implies that autophagy formation could limit therapeutic efficacies of CXCR4 antagonist. Our data also support targeting the CXCR4/SDF-1 signaling axis with autophagy inhibitors could increase efficacies of current therapies.
Acknowledgments
Z.C performed the experiments and analyzed the data. A.T performed the experiments. N.M planned the experiments and wrote a manuscript.
Supports
N.M is supported by Research Scholar Award (American Cancer Society) and 1R01CA181319 (NCI/NIH).
Footnotes
Authors declare no conflict of interests.
References
- 1.Leonard JP, Williams ME, Goy A, Grant S, Pfreundschuh M, Rosen ST, et al. Mantle cell lymphoma: biological insights and treatment advances. Clin Lymphoma Myeloma. 2009;9:267–77. doi: 10.3816/CLM.2009.n.055. [DOI] [PubMed] [Google Scholar]
- 2.Salaverria I, Perez-Galan P, Colomer D, Campo E. Mantle cell lymphoma: from pathology and molecular pathogenesis to new therapeutic perspectives. Haematologica. 2006;91:11–6. [PubMed] [Google Scholar]
- 3.Weigert O, Unterhalt M, Hiddemann W, Dreyling M. Mantle cell lymphoma: state-of-the-art management and future perspective. Leuk Lymphoma. 2009;50:1937–50. doi: 10.3109/10428190903288514. [DOI] [PubMed] [Google Scholar]
- 4.Adams JM, Harris AW, Strasser A, Ogilvy S, Cory S. Transgenic models of lymphoid neoplasia and development of a pan-hematopoietic vector. Oncogene. 1999;18:5268–77. doi: 10.1038/sj.onc.1202997. [DOI] [PubMed] [Google Scholar]
- 5.Lovec H, Grzeschiczek A, Kowalski MB, Moroy T. Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J. 1994;13:3487–95. doi: 10.1002/j.1460-2075.1994.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirt C, Schuler F, Dolken L, Schmidt CA, Dolken G. Low prevalence of circulating t(11;14)(q13;q32)-positive cells in the peripheral blood of healthy individuals as detected by real-time quantitative PCR. Blood. 2004;104:904–5. doi: 10.1182/blood-2004-02-0738. [DOI] [PubMed] [Google Scholar]
- 7.Fu K, Weisenburger DD, Greiner TC, Dave S, Wright G, Rosenwald A, et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106:4315–21. doi: 10.1182/blood-2005-04-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin P, Coleman M, Leonard JP. Progress in mantle-cell lymphoma. J Clin Oncol. 2009;27:481–3. doi: 10.1200/JCO.2008.19.5032. [DOI] [PubMed] [Google Scholar]
- 9.Sharma M, Afrin F, Satija N, Tripathi RP, Gangenahalli GU. Stromal-derived factor-1/CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev. 2011;20:933–46. doi: 10.1089/scd.2010.0263. [DOI] [PubMed] [Google Scholar]
- 10.Grignani G, Perissinotto E, Cavalloni G, Carnevale Schianca F, Aglietta M. Clinical use of AMD3100 to mobilize CD34+ cells in patients affected by non-Hodgkin's lymphoma or multiple myeloma. J Clin Oncol. 2005;23:3871–2. doi: 10.1200/JCO.2005.55.250. author reply 2-3. [DOI] [PubMed] [Google Scholar]
- 11.Clezardin P. Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res. 2011;13:207. doi: 10.1186/bcr2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng D, Vasquez-Medrano DA, Brown JM. Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br J Cancer. 2011;104:1805–9. doi: 10.1038/bjc.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell B, Mahalingam M. The CXCR4/CXCL12 axis in cutaneous malignancies with an emphasis on melanoma. Histol Histopathol. 2014 doi: 10.14670/HH-29.1539. [DOI] [PubMed] [Google Scholar]
- 14.Wu PF, Lu ZP, Cai BB, Tian L, Zou C, Jiang KR, et al. Role of CXCL12/CXCR4 signaling axis in pancreatic cancer. Chin Med J (Engl) 2013;126:3371–4. [PubMed] [Google Scholar]
- 15.Gangadhar T, Nandi S, Salgia R. The role of chemokine receptor CXCR4 in lung cancer. Cancer Biol Ther. 2010;9:409–16. doi: 10.4161/cbt.9.6.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger JA, Stewart DJ, Wald O, Peled A. Potential of CXCR4 antagonists for the treatment of metastatic lung cancer. Expert Rev Anticancer Ther. 2011;11:621–30. doi: 10.1586/era.11.11. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Yang J, Qian J, Li H, Romaguera JE, Kwak LW, et al. Role of the microenvironment in mantle cell lymphoma: IL-6 is an important survival factor for the tumor cells. Blood. 2012;120:3783–92. doi: 10.1182/blood-2012-04-424630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Kurtova AV, Tamayo AT, Ford RJ, Burger JA. Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): importance for interactions with the stromal microenvironment and specific targeting. Blood. 2009;113:4604–13. doi: 10.1182/blood-2008-10-185827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graf L, Iwata M, Torok-Storb B. Gene expression profiling of the functionally distinct human bone marrow stromal cell lines HS-5 and HS-27a. Blood. 2002;100:1509–11. doi: 10.1182/blood-2002-03-0844. [DOI] [PubMed] [Google Scholar]
- 21.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- 22.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–81. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 23.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–6. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Orlowski RZ, Wang M, Kwak L, McCarty N. Osteoblastic niche supports the growth of quiescent multiple myeloma cells. Blood. 2014 doi: 10.1182/blood-2013-07-517136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S, Law P, Francis K, Palsson BO, Ho AD. Symmetry of initial cell divisions among primitive hematopoietic progenitors is independent of ontogenic age and regulatory molecules. Blood. 1999;94:2595–604. [PubMed] [Google Scholar]
- 27.Lanzkron SM, Collector MI, Sharkis SJ. Hematopoietic stem cell tracking in vivo: a comparison of short-term and long-term repopulating cells. Blood. 1999;93:1916–21. [PubMed] [Google Scholar]
- 28.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–9. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Proteasome inhibition in the treatment of cancer. Cell Cycle. 2005;4:290–6. [PubMed] [Google Scholar]
- 31.Chen Z, Romaguera J, Wang M, Fayad L, Kwak LW, McCarty N. Verapamil synergistically enhances cytotoxicity of bortezomib in mantle cell lymphoma via induction of reactive oxygen species production. Br J Haematol. 2012;159:243–6. doi: 10.1111/bjh.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohshima-Hosoyama S, Davare MA, Hosoyama T, Nelon LD, Keller C. Bortezomib stabilizes NOXA and triggers ROS-associated apoptosis in medulloblastoma. J Neurooncol. 2011;105:475–83. doi: 10.1007/s11060-011-0619-0. [DOI] [PubMed] [Google Scholar]
- 33.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–8. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosich L, Xargay-Torrent S, Lopez-Guerra M, Campo E, Colomer D, Roue G. Counteracting autophagy overcomes resistance to everolimus in mantle cell lymphoma. Clin Cancer Res. 2012;18:5278–89. doi: 10.1158/1078-0432.CCR-12-0351. [DOI] [PubMed] [Google Scholar]
- 36.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–7. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 37.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–96. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–73. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipinski MM, Hoffman G, Ng A, Zhou W, Py BF, Hsu E, et al. A genome-wide siRNA screen reveals multiple mTORC1 independent signaling pathways regulating autophagy under normal nutritional conditions. Dev Cell. 2010;18:1041–52. doi: 10.1016/j.devcel.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–7. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothe K, Lin H, Lin KB, Leung A, Wang HM, Malekesmaeili M, et al. The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells. Blood. 2014;123:3622–34. doi: 10.1182/blood-2013-07-516807. [DOI] [PubMed] [Google Scholar]
- 42.do Carmo A, Patricio I, Cruz MT, Carvalheiro H, Oliveira CR, Lopes MC. CXCL12/CXCR4 promotes motility and proliferation of glioma cells. Cancer Biol Ther. 2010;9:56–65. doi: 10.4161/cbt.9.1.10342. [DOI] [PubMed] [Google Scholar]
- 43.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 44.Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, et al. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62:5930–8. [PubMed] [Google Scholar]
- 45.Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–24. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–51. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rausch V, Liu L, Apel A, Rettig T, Gladkich J, Labsch S, et al. Autophagy mediates survival of pancreatic tumour-initiating cells in a hypoxic microenvironment. J Pathol. 2012;227:325–35. doi: 10.1002/path.3994. [DOI] [PubMed] [Google Scholar]
- 48.Kongara S, Karantza V. The interplay between autophagy and ROS in tumorigenesis. Front Oncol. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Ishdorj G, Gibson SB. Reactive oxygen species regulation of autophagy in cancer: implications for cancer treatment. Free Radic Biol Med. 2012;53:1399–410. doi: 10.1016/j.freeradbiomed.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan G, Xie W, Liu Z, Xu W, Lao Y, Huang N, et al. Hypoxia-induced MIR155 is a potent autophagy inducer by targeting multiple players in the MTOR pathway. Autophagy. 2014;10:70–9. doi: 10.4161/auto.26534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, et al. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72:1773–83. doi: 10.1158/0008-5472.CAN-11-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh YS, Kim HY, Song IC, Yun HJ, Jo DY, Kim S, et al. Hypoxia induces CXCR4 expression and biological activity in gastric cancer cells through activation of hypoxia-inducible factor-1alpha. Oncol Rep. 2012;28:2239–46. doi: 10.3892/or.2012.2063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.