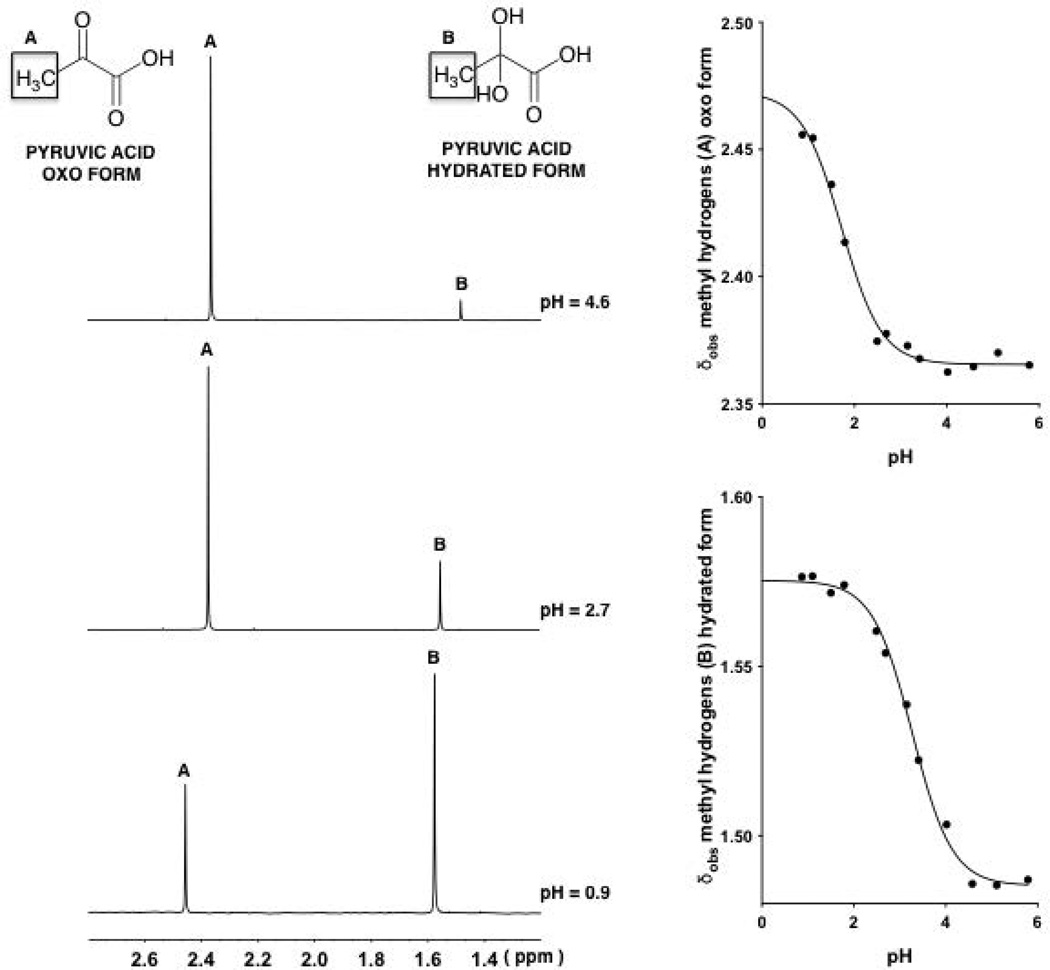
Figure 1.
1H-NMR spectra (9:1 v/v H2O:D2O)of the methyl resonances of pyruvic acid (1, oxo form and its hydrate) at various pH values, 4.6, 2.7 and 0.9 (top to bottom on the left).Observed chemical shifts (δobs) of the signals arising from the methyl hydrogens associated with the oxo and hydrated forms of 1 plotted against pH (right). The two continuous lines with a sigmoidal shape are described by Eq. 14, fitting δobs values at different pH values.