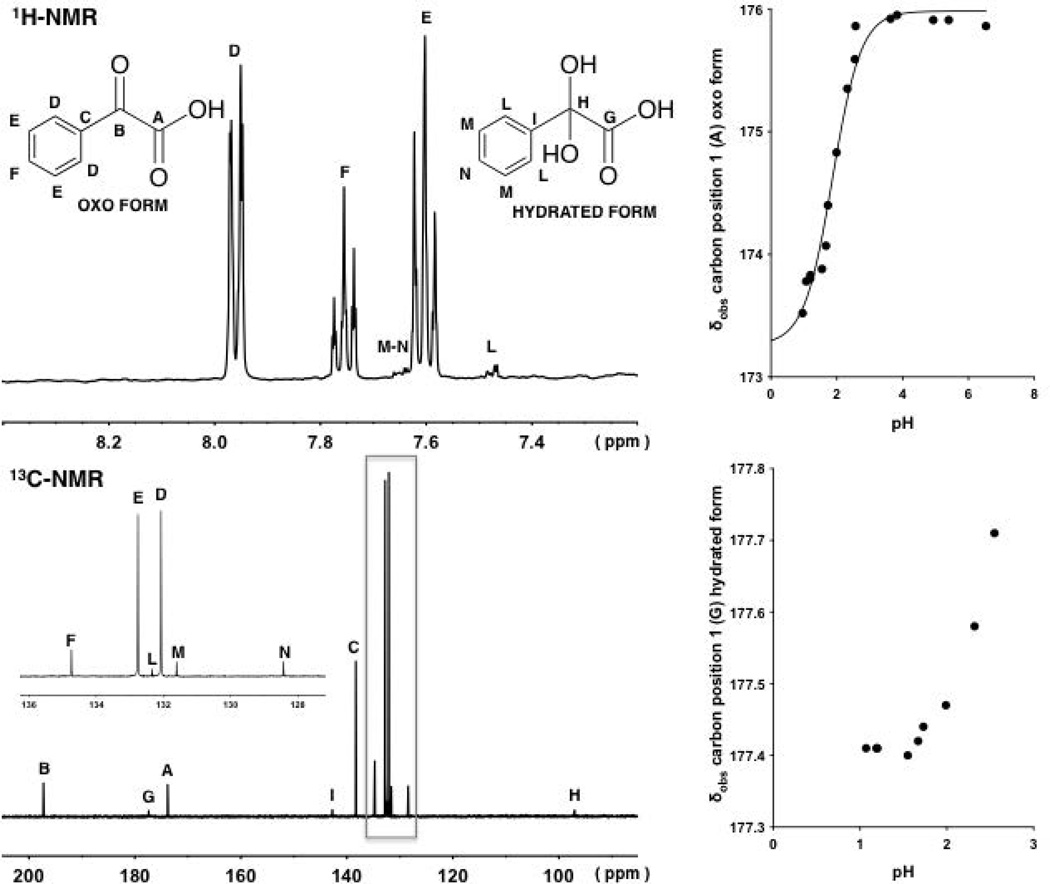
Figure 6.
1H-NMR and 13C-NMR of compound 4 at pH values at approximately 1.5 and 1.2, respectively (left). Variation of the observed chemical shifts (δobs) of the signals arising from the carbon in position 1 associated with the oxo and hydrated forms of 4 at different pH values (right).The continuous line with a sigmoidal shape is described by Eq. 14, fitting δobs values at different pH values.