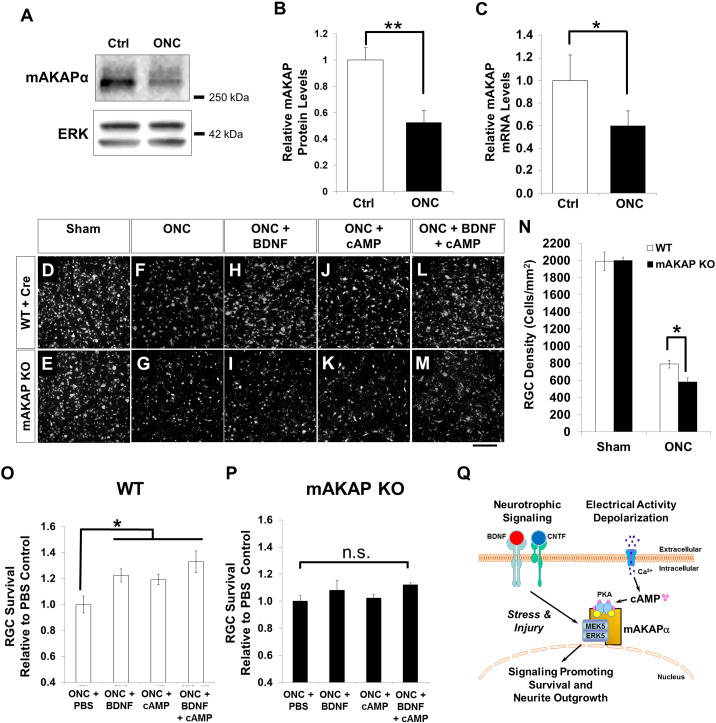
Fig. 4.
Loss of mAKAP exacerbates RGC cell death after optic nerve injury. (A) Western blot for mAKAP and ERK1/2 protein in uninjured RGCs and 3 days after optic nerve crush (ONC). (B) Quantitation of protein expression revealed a 40–50% reduction in RGC mAKAPα levels compared to uncrushed controls (N = 3). (C) Quantitative PCR demonstrating a similar reduction in retinal mAKAPα mRNA expression 3 days after crush injury (n = 3). (D–P) Induced mAKAP knockout (KO) in the adult suppresses RGC survival after optic nerve injury. AAV2-cre was injected intravitreally in mice 2 weeks prior to optic nerve crush. RGCs were retrogradely labeled with fluorogold 1 week prior to optic nerve injury. (D–M) Representative images of flat-mounted retinal segments in different conditions as marked. Scale bar represents 100 μm. (N–P) Quantification of RGC survival. A similar number of RGCs was observed in WT and mAKAP KO mice (mAKAPfl/fl animals with AAV2-cre injection) following sham optic nerve injury (exposed optic nerve, without crush). By one week after optic nerve crush, however, significantly fewer fluorogold-labeled RGCs were observed in mAKAP knockout animals than control animals (N). Application of BDNF and cAMP significantly increased RGC survival after optic nerve injury in WT animals (O), but had no effect on mAKAP KO mice (P) (n = 4 mice per group). *P < 0.05, **P < 0.01; one-way analysis of variance; error bars indicate standard error of mean. (Q) Model for mAKAPα-dependent signaling in stressed RGCs. mAKAPα can organize signalosomes that contain signaling molecules such as ERK5 and PKA that are responsive to upstream neurotrophin and electrical signals and that we propose will induce gene expression promoting both RGC survival and neurite outgrowth following injury.