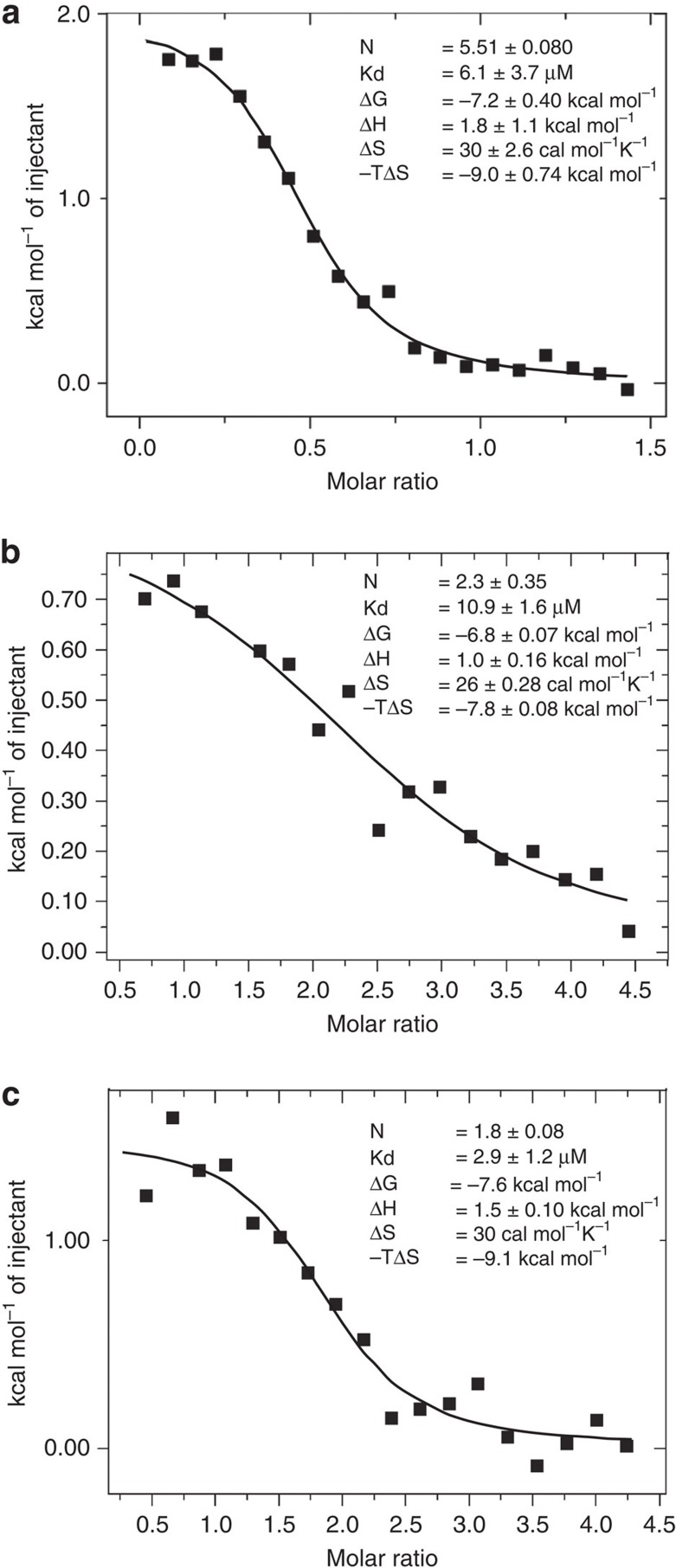
Figure 6. Isothermal titration calorimetry analysis of Hb binding to KdHpuA.
(a) Multiple 2 μl injections of 1.3 mM Hb were titrated into 170 μM KdHpuA. Values given for the stoichiometry, affinity and thermodynamics of the interaction are means and s.d. of five independent experiments. (b) The reverse experiment to a—2 μl injections of wild-type KdHpuA at 600 μM were titrated into Hb at 24 μM. Binding values are means and s.d. of two independent experiments. (c) KdHpuA at 800 μM titrated into 39 μM Hb:Hp(1–1), values are calculated from fitting to a single experiment. In all cases a similar titration of injectant into buffer was subtracted before analysis. (See Supplementary Fig. 10 for the raw data.).