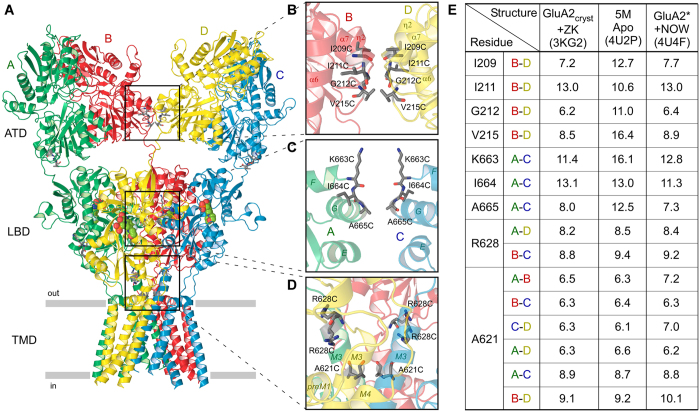
Figure 1. Location of substituted cysteines and distances between them.
(A) Ribbon diagram of the GluA2cryst structure in complex with competitive antagonist ZK200775 (PDB ID: 3KG2). Four subunits (A–D) are in different colors. (B–D) Close-up views of intersubunit interfaces between two ATD dimers (B), two LBD dimers (C) and LBD-TMD linkers and ion channel domains (D). Residues substituted with cysteines are shown as sticks. The ribbon diagram is semitransparent. In c, the subunits B and D are removed for clarity. (E) Table showing distances (in Å) between Cα’s of residues substituted with cysteines and measured in three selected structures: GluA2cryst in complex with ZK200775 (PDB ID: 3KG2), 5M construct in the apo state (PDB ID: 4U2P) and GluA2* in complex with partial agonist NOW (PDB ID: 4U4F). For R628 and A621, the distances are shown for more than one pair of residues that belong to different pairs of subunits.