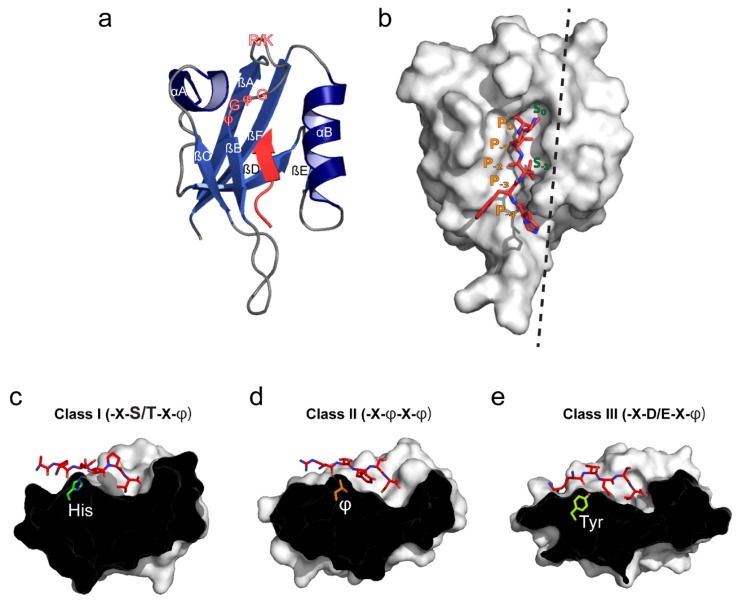
Figure 1.
Structural overview of the canonical PDZ domain fold, ligand binding and peptide-based classification. (a) The canonical PDZ domain fold comprises two α-helices (αA and αB) and six β-strands (βA–βF) (PDB: 2LUI [22]). The ligand is shown in red and is stitched into the binding groove located between the αB helix and βB strand. The peptide/protein-binding groove is composed by the R/K-X-X-X-G-φ-G-φ (X—any residue; φ—hydrophobic residue) loop indicated in red outlined text; (b) Surface view of the canonical PDZ domain. Hydrophobic pockets S0 and S-2 (for class II domains) are indicated in green. Correspondingly, the five C-terminal ligand residues are denoted from P0 to P−4 in orange. The black dashed line indicates the cross section plane used in panels c-e; (c) Class I binding domains usually recognize ligands with either Ser or Thr at P−2. The αB1 residue is usually His (PDB: 1TP3 [23]); (d) Class II binding domains recognize ligands with hydrophobic residues at P−2. The αB1 residue is often hydrophobic and allows for formation of a second hydrophobic binding pocket (denoted S-2 in panel b) (PDB: 1N7F [24]); (e) Ligands binding class III domains typically have Asp or Glu at P−2 and Tyr at the αB1 position (PDB: 1B8Q [25]).