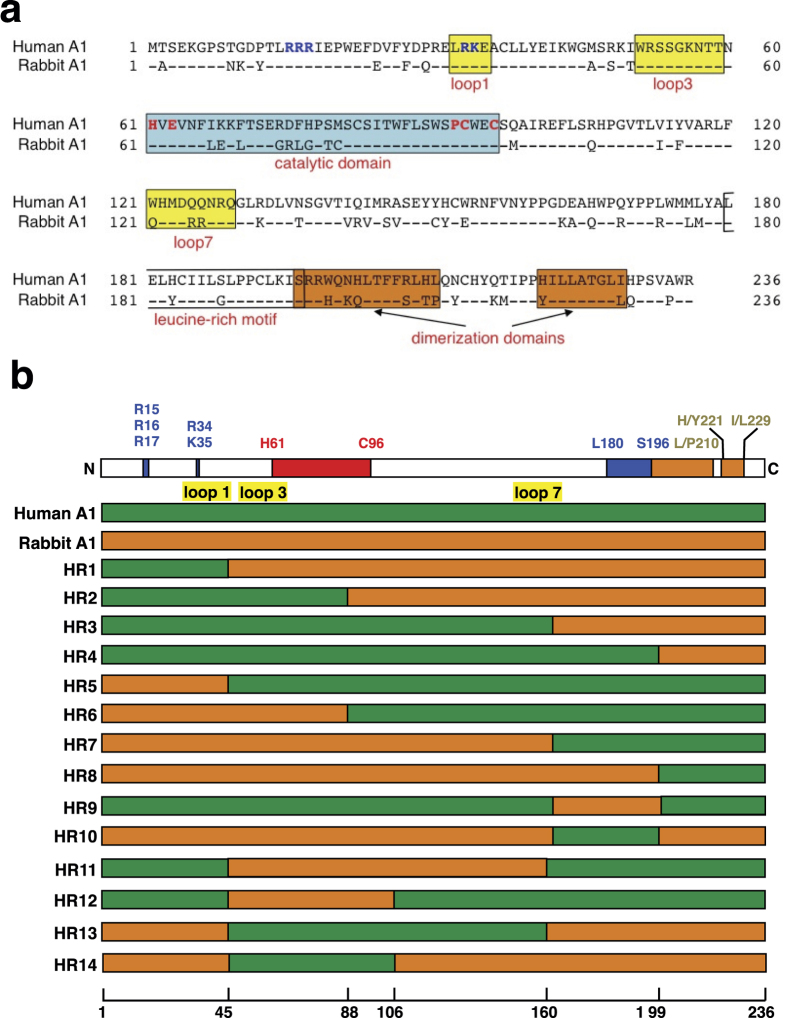
Figure 1. Generation of chimeric molecules from human and rabbit A1.
(a) A comparison of the predicted amino acid sequences of human and rabbit A1 proteins. Amino acid sequence alignment of A1 from human (GenBank accession number: NM001644) and rabbit (U10695) generated with ClustalW software. The numbers are amino acid residue positions. Identical amino acids are shown with a single dot. The putative bipartite nuclear targeting signals (R15, R16, R17, R33, and R34) are shown in blue. The H61, E63, P92, C93, and C96 residues at the catalytic domain (blue box), which play essential roles in catalysis and Zn2+-coordination, are indicated in red. The additional loop structures (loops 1, 3, and 7), predicted to form between the α-helices and β-sheet, have been implicated in the proteins’ interactions with their nucleic acid substrates (yellow boxes). The leucine-rich motif (L180–S196) (clear box) and two dimerization domains (S196–L/P210and H/Y221–I/L229) (orange boxes) are indicated. (b) Schemes of human A1, rabbit A1, and their derived chimeras. The domain organization of A1 is shown on top of the panel. The green bars correspond to regions from human A1, and the orange bars indicate regions from rabbit A1. The numbers indicate the amino acids of A1 that are replaced in each chimera, and correspond to the A1 amino acids (bottom).