Abstract
OBJECTIVES
Lower rates of cancer in the oldest old and in nursing home populations may reflect the increasing prevalence of frailty and a diminished capacity to sustain cancer cell growth and proliferation. This study aimed to determine cancer incidence in the frail relative to non-frail community resident older adults.
MATERIALS AND METHODS
Data come from 3,969 participants free of diagnosed cancer at the sixth follow up from three sites of the Established Populations for Epidemiologic Studies of the Elderly (EPESE), a population-based cohort study. Frailty status was determined from physical performance testing and self reported dependency in activities of daily living. Cancer incidence over the four subsequent years was identified through linkage with Medicare claims data. Logistic regression was used to estimate the odds of cancer incidence with respect to frailty status in multiple models with progressive adjustment for covariates.
RESULTS
Of the 3,969 participants, 1,340 (33·8%) were identified as frail. Cancer incidence at 4 years was lower in frail participants overall (OR 0·64; 95% CI 0·46–0·89) and frail men in particular (OR 0·54; 95% CI 0·33–0·87). Incidence was lower in women (3.7%) than men (8.8%), but was not lower in frail women compared with non-frail women (OR 0·77; 95% CI 0·48 –1·23).
CONCLUSION
Frailty status was associated with decreased cancer incidence, particularly in men, and suggests that mechanisms related to the pathogenesis of frailty may also play a role in inhibiting tumorigenesis. Why this would be more apparent in men than women remains to be clarified.
Keywords: frailty, cancer, incidence, cellular senescence, microenvironment, elderly
Cancer incidence increases from the third through eighth decades, but for unclear reasons, declines sharply thereafter (1). Cancers that occur in the oldest-old patients are often described as less aggressive with slower growth, less prominent vessels and fewer metastases than those arising in younger persons (2–5). In addition, animal studies have implicated tumor suppressor mechanisms in promoting cellular senescence and the development of an aging phenotype(6). These observations provide the basis for the argument that tissues in older individuals may be less capable of promoting tumor growth, perhaps by virtue of lack of proliferation-potentiating growth factors and altered cytokine profile (7).
A general feature of aging, particularly in humans, is expanded heterogeneity evident in almost all clinical and social parameters (8). Some 75 year olds appear robust and highly functioning, whereas others are exceedingly frail. In general, the prevalence of frailty increases with advancing age and the factors that account for this considerable variability are the focus of current investigation. Although frailty is associated with increased co-morbid illness, (9–11) and functional limitations, including deficiencies in basic activities of daily living (ADL), frailty also may occur in the absence of known co-morbidity (12). Further, some who meet criteria for frailty may still maintain functional capacity to perform ADL (12).
For the purposes of clinical investigation, several operational definitions of frailty have been proposed with most encompassing assessment of functional impairment, muscle strength and body composition (12). The pathogenesis of frailty remains poorly understood, but numerous endocrine and nutritional factors have been implicated, and it appears that senescence and apoptotic pathways may play a crucial role (13–16). Those who are ‘frail’ have decreased physiologic reserve, diminished resistance to stressors, increased vulnerability to adverse outcomes, and a high risk of death (17). Thus, ‘frailty’ may be considered a phenotype that develops in some people as a consequence of chronic disease and in others simply as a consequence of aging.
In our previous work (18–20), we contend that the tissue microenvironment in frail individuals may reflect overall deregulated homeostasis, as observed with cellular senescence, and as such, is less likely to be conducive to tumor cell proliferation. Supporting this notion, we observed strikingly less cancer in older patients in the nursing home when compared to age-matched individuals living in the community (21). However, because the nursing home environment does not foster preventative screening and comprehensive diagnostic evaluations, it is possible that lower cancer rates observed reflect under-diagnosis and not necessarily lower cancer incidence. To address this issue, we examined cancer incidence among frail and non-frail largely community-dwelling individuals participating in the Established Populations for Epidemiologic Studies of the Elderly (EPESE) over a four year time span.
Methods
Study Population
The EPESE consists of prospective epidemiologic studies of approximately 14,000 persons 65 years of age and older in four different communities: East Boston, Massachusetts; two rural counties in Iowa; New Haven, Connecticut; and segments of five counties in the north-central Piedmont area of North Carolina. The study design included an initial baseline household interview followed by continued surveillance of morbidity and mortality (22). Participants were re- contacted annually in conjunction with the collection of data on cause of death and factors related to hospitalization and nursing home admissions. At the sixth follow-up (7th year of the study), an in-home interview was conducted and data on self reported functional status and performance-based measures were collected. This was the first examination to include physical performance measures, which are critical components of the assessment of frailty (see below). Thus, follow-up 6 of EPESE served as the baseline for the current research and the inception point for subsequent linkage to Medicare claims data to identify incident cancer cases.
Sample
The study utilized data from three of the four of the EPESE sites: East Boston, a blue-collar community composed of low- and middle-income working-class persons, most of whom were Italian American; two rural counties in east central Iowa where agriculture is the major industry; and New Haven, where the largest employers are educational institutions, manufacturing, and service industries. The sampling frame for each site differed, and EPESE was not intended to generate population-based estimates. East Boston employed a total community census whereas Iowa used a private census of the area supplemented by lists of elderly persons compiled by the Area’s Agency on Aging. New Haven conducted a stratified cluster sampling of three different types of residents: those dwelling in public housing, in private housing for the elderly, and elsewhere in the community. Participation rates ranged from 76% to 89% across sites and subgroups (23, 24).
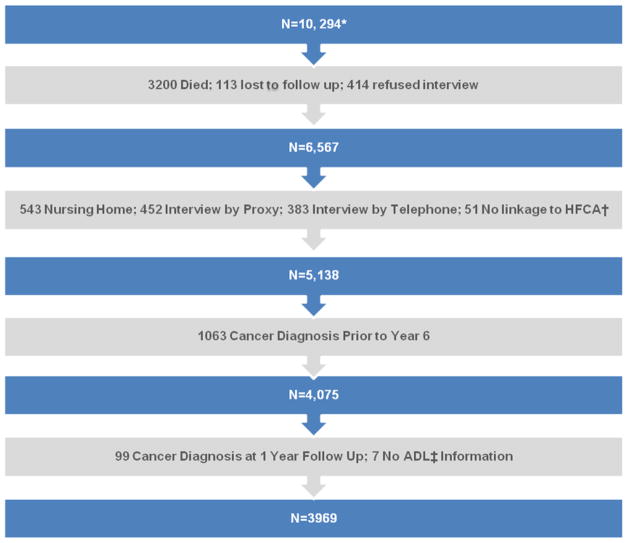
At the initial baseline interview there were 10,294 participants aged 65 and older (figure 1). At the time of the sixth follow-up conducted in 1988 (the baseline for the current study), 3,200 participants were known to have died, 113 were lost to follow-up, and 414 refused to be interviewed. Of the remaining 6,567 persons, 1,378 were excluded because we were unable to obtain any physical performance measures (543 resided in a nursing home, 452 had their interview through a proxy, and 383 were interviewed by telephone), and an additional 51 could not be linked to Health Care Financing Administration data to obtain Part A Medicare costs. Among the remaining 5,138 participants, we excluded 1063 participants who had a diagnosis of cancer prior to 1988 and 99 who were diagnosed with cancer within 12 months of the sixth follow-up (baseline) and 7 who did not have information on ADL. Accordingly, for the purposes of this analysis, a new diagnosis of cancer occurring from July 1988 through June 1992 were considered ‘incident’ cases.
Fig. 1.
Stepwise Ascertainment of the Study participants from the Baseline EPESE** Population
*Participants aged 65 years and older
**Established Populations for Epidemiologic Studies of the Elderly (EPESE)
†Health Care Financing Administration
‡Activities of Daily Living
Measurements
All measures except cancer incidence data were obtained from the EPESE data set.
Co morbidities, functional and vital status
Prevalent heart disease, stroke, diabetes, hypertension, and arthritis were ascertained from participant self-report of ever being told by a doctor that they had the condition. It is from self-report, that we capture dependency with ADL, namely the need for help with, bathing, transferring from bed to chair, dressing, eating, or using the toilet.. Information on vital status came from obituaries, contact with proxies, and the National Death Index.
Smoking history
Subjects who reported smoking cigarettes within the prior 10 years were considered to have positive history for smoking. Those who never reported smoking or who quit more than 10 years prior to evaluation were considered as having a negative smoking history or non-smokers.
Cancer diagnosis
Incident cancer over the subsequent 4 years after baseline assessment was derived from Medicare files which included all hospital admissions (Part A). The inpatient claims provide date of admission and discharge, as well as diagnostic and procedure codes using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) for each admission. Incident cases were identified as those who had a diagnosis of cancer including carcinoma-in-situ (ICD, codes 140.0–239.0, including carcinoma-in-situ 230.0– 234.0) in any of the five diagnostic positions contained in the record. Within this we excluded benign neoplasms (210–229) and the category ‘other malignant neoplasm’s of the skin’ (173.0–173.9) and carcinoma-in-situ of skin (232.0–232.9). Diagnosis within one year and four years of follow-up was based on admission date.
Participants were considered to have a prior history of cancer if they reported ever being told by a doctor that they had cancer in any of the previous interviews including the sixth follow-up conducted in 1988 (the baseline for the current study).
Physical performance testing
To test walking speed, an 8-foot walking course, with no obstructions for an additional 2 feet at either end, was denoted by placing a rigid 8-foot carpenter’s rule to the side of the course. Participants were instructed to “walk to the other end of the course at your usual speed, just as if you were walking down the street to go to the store.” Participants could use assistive devices if needed, and each participant was timed for two walks. The faster of the two times was used in the analysis. To test the ability to rise from a chair without using one’s arms, a straight-backed chair was placed next to a wall; participants were asked to fold their arms across their chest and to stand up from the chair one time. If successful, participants were asked to stand up and sit down five times as quickly as possible, and were timed from the initial sitting position to the final sitting position at the end of the fifth stand.
Categories of performance ranging from 0 to 4 were created for each performance measure to permit analyses that included those unable to perform the task to whom a score of 0 was assigned (25). We further categorized participants with a score of 0 on the chair stand test or a score of 0 or 1 (usual gait speed < .44 meters/second) on the walking speed test to have poor physical performance consistent with frailty.
Frailty Definition
Since information was unavailable for some components of the most commonly used operational definition of frailty (12), we used physical performance findings as has been described previously by Gill and colleagues (26). Further, to minimize misclassification of the non-frail, we also incorporated reported ADL dependency. Thus, participants who scored 0 on the chair stand test or had a walking speed score of 0 or 1 or had reported dependency in any ADL as defined above, were classified as frail. All other participants were considered non-frail.
Statistical analysis
All analyses were done for the overall population and for men and women separately. Descriptive statistics were used to evaluate differences in study population characteristics by frailty status. Cancer incidence and all-cause mortality after 4 years was analyzed with respect to presence or absence of frailty at baseline as defined above. We estimated multiple conditional logistic-regression models to assess the independent association of frailty with incident cancer at follow up. Variables typically associated with cancer incidence and other potential confounders including age, sex (overall population only), demographics, smoking history, chronic diseases and death during follow up were introduced in successive models. We obtained estimates of odds ratios from the logistic models.
Results
The study population (n=3,969, Figure 1) consisted of all participants who were alive and interviewed in person at follow up visit 6, who had no prior diagnosis of cancer or evidence of cancer development within the next year, had complete information on ADL dependency to assess frailty status, and for whom linkage to Medicare Claims data was available. The population included 1372 (34·6%) men and 2597 (65·4%) women. Of these, 334 men and 902 women were frail by physical performance criteria; an additional 35 men and 49 women with complete performance data were frail by ADL dependency criteria alone. Among the 42 men and 97 women missing performance test findings, six and 14, respectively met ADL dependency frailty criteria and were included in the frail population. Applying this operational definition of frailty yielded 1,340 (33·8%) frail participants overall. Frailty was more common in women than men (37·1% versus 27·3%; p<0·001).
Table 1 depicts the baseline characteristics of the population overall and stratified by sex. As shown, the proportion of frail individuals was greater with advancing age and highest in the age group 85 years and older. Women tended to be identified as frail at earlier ages than men. Frailty was also higher in African Americans overall and in both sexes. Frail women were more likely to be non-smokers than non-frail women. Frailty was least prevalent in Iowa and most prevalent in New Haven with nearly 50% of women classified as frail.
Table 1.
Baseline characteristics of the study population according to frailty status stratified by sex
| Characteristic (n) | Total n=3969 | Men n=1372 | Women n=2597 |
|---|---|---|---|
| Frail % (n) | Frail % (n) | Frail % (n) | |
| Age, y | |||
| 70–74 (1113) | 22.7(253) | 21.0(91) | 23.8(162) |
| 75–79 (1426) | 29.3(418) | 23.5(123) | 32.7(295) |
| 80–84 (839) | 39.6(332) | 33.9(85) | 42.0(247) |
| ≥85 (591) | 57.0(337) | 46.3(76) | 61.1(261) |
| Race | |||
| Black (211) | 51.7(109) | 45.6(31) | 54.5(78) |
| Not Black (3758)* | 32.8(1231) | 26.4(344) | 36.1(887) |
| Study Site | |||
| East Boston (1375) | 35.7(491) | 27.7(132) | 40.0(359) |
| Iowa (1607) | 26.3(422) | 22.7(119) | 28.0(303) |
| New Haven (987) | 43.3(427) | 33.5(124) | 49.1(303) |
| History of smoking | |||
| Yes(787) | 32.0(252) | 30.6(110) | 33.2(142) |
| No(3182) | 34.2(1088) | 26.2(265) | 37.9(823) |
| Prevalent Chronic Diseases | |||
| MI(609) | 40.2(245) | 29.7(84) | 49.4(161) |
| Stroke(325) | 42.6.9(169) | 47.9(69) | 55.2(100) |
| Diabetes(707) | 31.9(301) | 31.9(81) | 48.6(220) |
| Hypertension(2160) | 38.3(828) | 31.3(203) | 41.3(625) |
| Arthritis(2089) | 34.4(1370) | 28.0(181) | 37.3(538) |
| Vital Status | |||
| Dead± (699) | 59.9(370) | 44.0(147) | 61.1(223) |
Includes Caucasians and Hispanics
Denotes % frail among those with specific chronic conditions
Follow up 4 years
Abbreviations: MI, Myocardial infarction
Hypertension was the most prevalent condition in all groups, present in more than 50% of the total population with a slightly higher percentage in frail individuals (61·8% versus 50·7%). Stroke (10·5% versus 7·0%) and MI (20·6% versus 12·6%) were present in greater percentages in men; whereas hypertension (58·2% versus 47·2%) and, arthritis (55·5% versus 47·2% respectively) were more frequent in women (data not shown in the table).
Incidence of cancer in the total population was 5·5%, and was higher in men than women (8·8% versus 3·7%; p<.0·001). In unadjusted analysis, cancer incidence in the overall population did not differ between the frail and non-frail although there was a trend in that direction (4·5% versus 6·0%; p=0·06). Sex-stratified analysis revealed a trend toward lower cancer incidence in frail men (9·5% in non-frail and 6·9% in frail; p=0·12). Deaths during follow up were higher in men (24·3%) than women (14·0%) and occurred with greater frequency among the frail subgroup in both sexes (Table 2).
Table 2.
Cancer Incidence and Total Mortality at Four Years by Baseline Frailty Status
| Characteristic | Frail %(n) | Non-Frail %(n) | ||||
|---|---|---|---|---|---|---|
| Total | Men | Women | Total | Men | Women | |
| Cancer Incidence | 4.5(61) | 6.9(26) | 3.6(35) | 6.0(157) | 9.5(95) | 3.8(62) |
| Death | 27.6(370) | 39.2(147) | 23.1(223) | 12.5(329) | 18.8(187) | 8.7(142) |
Table 3 shows the odds of developing cancer associated with frailty as estimated by successive models. In the simple age-adjusted model (Model 1) there was a trend towards frailty as being protective of cancer in men only. After adjusting for demographic factors, race, in particular, and smoking status (Model 2), a protective relationship between frailty and cancer incidence emerged in men (OR=0·59; 95% CI=0·37–0·94). Further adjustment for chronic diseases in Model 3 did not attenuate the strength of association between frailty and decreased cancer incidence in men. Incorporating death during follow up, in the final model strengthened the association in men and also revealed a significant association between frailty and cancer in the overall population. However, no association between frailty and cancer was seen in any of these models in women, in whom the rate of cancer incidence was quite low.
Table 3.
Association between Cancer Incidence and Frailty Overall and for Men and Women Separately
| Characteristic | Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Total | ||||
| Frailty | 0.80(0.58–1.09) | 0.77(0.56–1.05) | 0.77(0.56–1.06) | 0.64(0.46–0.89) |
| Age | 1.08(0.94–1.24) | 1.13(0.98–1.30) | 1.12(0.97–1.29) | 1.02(0.88–1.18) |
| Female Sex | 0.40(0.31–0.53) | 0.42(0.32–0.56) | 0.42(0.32–0.56) | 0.50(0.37–0.66) |
| Black Race | 1.33(0.75–2.40) | 1.35(0.75–2.42) | 1.34(0.74–2.43) | |
| East Boston | 1.12(0.83–1.50) | 1.23(0.75–1.70) | 1.12(0.74–1.70) | |
| Smoking History | 1.67(1.22–2.28) | 1.64(1.20–2.24) | 1.49(1.08–2.04) | |
| MI | 1.18(0.83–1.69) | 1.08(0.75–1.55) | ||
| Stroke | 0.10(0.60–1.64) | 0.91(0.55–1.51) | ||
| Diabetes | 0.73(0.49–1.10) | 0.67(0.44–1.00) | ||
| Hypertension | 1.07(0.81–1.42) | 1.02(0.76–1.36) | ||
| Arthritis | 0.99(0.66–1.46) | 0.98(0.66–1.45) | ||
| Died During Follow-up | 3.13(2.29–4.27) | |||
| Men | ||||
| Frailty | 0.64(0.40–1.00) | 0.59(0.37–0.94) | 0.61(0.38–0.97) | 0.54(0.33–0.87) |
| Age | 1.29(1.07–1.56) | 1.34(1.10–1.62) | 1.33(1.09–1.61) | 1.25(1.03–1.53) |
| Black Race | 2.11(1.02–4.38) | 2.21(1.05–4.62) | 2.15(1.02–4.52) | |
| East Boston | 1.14(0.76–1.70) | 1.29(0.73–2.26) | 1.28(0.73–2.26) | |
| Smoking History | 1.44(0.95–2.19) | 1.44(0.95–2.19) | 1.36(0.89–2.08) | |
| MI | 1.34(0.86–2.08) | 1.27(0.81–1.98) | ||
| Stroke | 0.60(0.28–1.28) | 0.58(0.27–1.23) | ||
| Diabetes | 0.63(0.36–1.11) | 0.61(0.35–1.08) | ||
| Hypertension | 1.21(0.83–1.78) | 1.19(0.81–1.75) | ||
| Arthritis | 1.15(0.68–1.96) | 1.13(0.66–1.92) | ||
| Died During Follow-up | 1.97(1.30–2.99) | |||
| Women | ||||
| Frailty | 1.03(0.66–1.59) | 1.03(0.67–1.60) | 1.01(0.65–1.58) | 0.77(0.48–1.23) |
| Age | 0.87(0.70–1.07) | 0.92(0.74–1.14) | 0.92(0.74–1.14) | 0.81(0.65–1.01) |
| Black Race | 0.70(0.25–1.98) | 0.69(0.24–1.96) | 0.71(0.25–2.06) | |
| Study Site | 1.04(0.66–1.60) | 0.95(0.51–1.77) | 0.94(0.50–1.77) | |
| Smoking History | 2.04(1.28–3.25) | 2.01(1.25–3.23) | 1.72(1.06–2.79) | |
| MI | 0.91(0.49–1.72) | 0.82(0.43–1.57) | ||
| Stroke | 1.90(0.98–3.70) | 1.52(0.76–3.02) | ||
| Diabetes | 0.88(0.49–1.57) | 0.73(0.40–1.31) | ||
| Hypertension | 0.96(0.63–1.47) | 0.87(0.56–1.33) | ||
| Arthritis | 0.85(0.47–1.54) | 0.85(0.47–1.55) | ||
| Died During Follow-up | 5.45(3.43–8.68) | |||
Discussion
Using data from the prospective EPESE cohort residing in the community we found an independent negative relationship between frailty and cancer incidence in men, but not women who had an overall low rate of cancer. Taking into account the occurrence of death strengthened the apparent protective association between frailty and cancer and signifies that among persons who died, those who were frail at baseline were less likely to die from or with cancer than their more robust counterparts. This finding in the overall population and in men supports our original hypothesis.
In the present study we were unable to demonstrate an association between frailty and cancer incidence in women. However, this study does yield an important result as it demonstrates there is a higher prevalence of frailty in women, but lower cancer incidence relative to men, which also provides support for frailty as a protective mechanism. This result is consistent with the findings in the extant literature.(8)
There are some limitations in to the current study. First, we had to rely on a less commonly used operational definition for frailty since a few key components, such as grip strength, were not assessed in the EPESE. However, multiple alternative methods have successfully identified older individuals at increased risk of adverse outcomes (27). Gill and colleagues used the rapid-gait test (3 M walk) and the chair stand test to identify moderately to severely frail individuals for intervention (26). In a recent analysis of the EPESE database, it was determined that combined self reported functional status and physical performance testing was a better predictor of mortality in community-dwelling older persons than either method alone (28).
Secondly, we used hospitalization data to calculate cancer incidence, which may have resulted in reduced capture of incident cases. If we assume tumors in frail elderly are biologically less aggressive, then frailty-associated reduced cancer incidence may actually be more appropriately considered frailty-associated reduced cancer latency, as slow growing tumors with non-aggressive characteristics may lie below the threshold for clinical detection. Notably, studies have shown that persons with poor health are more likely to be referred for screening than the general population (29). In addition, Medicare claims data has been shown to compare well with the SEER (Surveillance Epidemiology and End Results) registry for identifying incident cancer cases in the elderly; differing by less than 6% for breast, lung, and colon cancer and identifying a greater percentage of prostate cancers (30). But, whether either of these applies to frail individuals is unknown. Finally, the generalizability of these findings to all races may be slightly limited due to the homogeneous nature of the EPESE population.
Some alternative explanations for the observed findings exist. It is possible that frail individuals with cancer were less likely to be diagnosed because of earlier death due to other causes. However, adjusting for death in our final model only strengthened this negative association between frailty and cancer. This suggests that although a higher percentage of frail elderly died, the cause of death was more likely due to reasons other than cancer.
Much has been written in recent years about the interface of cellular senescence and carcinogenesis (31–33). With each successive cellular division it is known that telomere length shortens and cellular levels of tumor suppressor genes increase (32). Both of these are features of cellular senescence wherein cellular proliferation is inhibited and cancer development becomes less likely. In murine (34) and human tissues there is an age-associated increase of these markers of cellular senescence, which promote the development of a tissue microenvironment (35) and phenotypic features (36, 37) as described in frail individuals. Given the same lifetime exposure to stressors, such as DNA or protein damage, that might produce a regenerative response and possibly neoplastic transformation in cells with replicative capacity, in senescent cells such a response and transformation is less likely to occur. Taking this one step further, given the same lifetime exposure to such stressors, it is likely that tissues with a greater component of senescent cells would be less cancer prone. However, direct clinical evidence of this phenomenon is lacking in humans. Data from the SEER registry clearly show that cancer incidence decreases in the older age groups and do not follow the Weibull hazard rate (38). Frailty modeling shows that this can be explained by heterogeneity in the risk of acquiring cancer in different individuals. Autopsy reports from studies done on the oldest old also demonstrate a lower cancer prevalence and presence of metastasis in persons aged over 95 years as compared to persons aged 75 to 95 (39). In a cross sectional analysis of the Medicare Beneficiary Survey we demonstrated that among persons aged 85 years or older, the prevalence of all types of cancer (excluding skin) in nursing home residents was approximately five times less than in persons of the same age residing in the community (21).
Our study provides the first evidence that mechanisms linking tumor suppression and aging similar to that observed in animals might also be active in frail individuals. Yet, it remains to be determined whether frail individuals have a greater proportion of resident senescent cells within their tissues or organs. If such were the case, this alone might account for the observed lower rate of cancer seen in the current study and also explain the decreased cancer incidence in the oldest old.
Acknowledgments
This work was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. A portion of this support was through a research and development contract with Medstar Health Research Institute.
The authors thank Dr. Jack Guralnik (Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging) for critical reading of the manuscript and suggestions. We also thank Ase Sewall and Carolyn Phillips for the provision of technological support.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altekruse SFKC, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site. 2010. [Google Scholar]
- 2.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92(7):550–6. doi: 10.1093/jnci/92.7.550. [DOI] [PubMed] [Google Scholar]
- 3.Ershler WB, Longo DL. Aging and cancer: issues of basic and clinical science. J Natl Cancer Inst. 1997;89(20):1489–97. doi: 10.1093/jnci/89.20.1489. [DOI] [PubMed] [Google Scholar]
- 4.Kreisle RA, Stebler BA, Ershler WB. Effect of host age on tumor-associated angiogenesis in mice. J Natl Cancer Inst. 1990;82(1):44–7. doi: 10.1093/jnci/82.1.44. [DOI] [PubMed] [Google Scholar]
- 5.Pili R, Guo Y, Chang J, Nakanishi H, Martin GR, Passaniti A. Altered angiogenesis underlying age-dependent changes in tumor growth. J Natl Cancer Inst. 1994;86(17):1303–14. doi: 10.1093/jnci/86.17.1303. [DOI] [PubMed] [Google Scholar]
- 6.Campisi J. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Exp Gerontol. 2003;38(1–2):5–11. doi: 10.1016/s0531-5565(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 7.Mascarucci P, Taub D, Saccani S, et al. Cytokine responses in young and old rhesus monkeys: effect of caloric restriction. J Interferon Cytokine Res. 2002;22(5):565–71. doi: 10.1089/10799900252982043. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the U.S. older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65B(2):246–55. doi: 10.1093/geronb/gbp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41(2):141–9. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310–7. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 11.Yancik R, Ershler W, Satariano W, Hazzard W, Cohen HJ, Ferrucci L. Report of the National Institute on Aging task force on comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62(3):275–80. doi: 10.1093/gerona/62.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51(1):245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 14.Kanapuru B, Ershler WB. Inflammation, Coagulation, and the Pathway to Frailty. The American Journal of Medicine. 2009;122(7):605–613. doi: 10.1016/j.amjmed.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 16.Fedarko NS. The biology of aging and frailty. Clin Geriatr Med. 2011;27(1):27–37. doi: 10.1016/j.cger.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried LP, Walston JD, Frailty Ferrucci L. In: Hazzard’s Geriatric Medicine and Gerontology. 6. Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, editors. New York: McGraw Hill Medical; 2009. pp. 631–646. [Google Scholar]
- 18.Denduluri N, Ershler WB. Aging biology and cancer. Semin Oncol. 2004;31(2):137–48. doi: 10.1053/j.seminoncol.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Ershler WB. Why tumors grow more slowly in old people. J Natl Cancer Inst. 1986;77(4):837–9. [PubMed] [Google Scholar]
- 20.Kaesberg PR, Ershler WB. The change in tumor aggressiveness with age: lessons from experimental animals. Semin Oncol. 1989;16(1):28–33. [PubMed] [Google Scholar]
- 21.Kanapuru B, Posani K, Muller D, Ershler WB. Decreased cancer prevalence in the nursing home. J Am Geriatr Soc. 2008;56(11):2165–6. doi: 10.1111/j.1532-5415.2008.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornoni-Huntley J, Ostfeld AM, Taylor JO, et al. Established populations for epidemiologic studies of the elderly: study design and methodology. Aging (Milano) 1993;5(1):27–37. doi: 10.1007/BF03324123. [DOI] [PubMed] [Google Scholar]
- 23.Cornoni-Huntley JBD, Ostfeld AM, Taylor JO, Wallace RB, editors. Established populations for epidemiologic studies of the elderly: resource data book. Washington, D.C: Government Printing Office; 1986. (NIH publication no. 86-2443.) [Google Scholar]
- 24.Cornoni-Huntley JBD, Lafferty ME, Everett DF, Brock DB, Farmer ME, editors. Established populations for epidemiologic studies of the elderly: resource data book. II. Washington, D.C: Government Printing Office; 1990. (NIH publication no. 90–495.) [Google Scholar]
- 25.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 26.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347(14):1068–74. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 27.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–9. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 28.Reuben DB, Seeman TE, Keeler E, et al. Refining the categorization of physical functional status: the added value of combining self-reported and performance-based measures. J Gerontol A Biol Sci Med Sci. 2004;59(10):1056–61. doi: 10.1093/gerona/59.10.m1056. [DOI] [PubMed] [Google Scholar]
- 29.Walter LC, Lindquist K, Covinsky KE. Relationship between health status and use of screening mammography and Papanicolaou smears among women older than 70 years of age. Ann Intern Med. 2004;140(9):681–8. doi: 10.7326/0003-4819-140-9-200405040-00007. [DOI] [PubMed] [Google Scholar]
- 30.McBean AM, Warren JL, Babish JD. Measuring the incidence of cancer in elderly Americans using Medicare claims data. Cancer. 1994;73(9):2417–25. doi: 10.1002/1097-0142(19940501)73:9<2417::aid-cncr2820730927>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113(2):160–8. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8(9):703–13. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 34.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melzer D, Frayling TM, Murray A, et al. A common variant of the p16(INK4a) genetic region is associated with physical function in older people. Mech Ageing Dev. 2007;128(5–6):370–7. doi: 10.1016/j.mad.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyner SD, Venkatachalam S, Choi J, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415(6867):45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 38.Svensson E, Moger TA, Tretli S, Aalen OO, Grotmol T. Frailty modelling of colorectal cancer incidence in Norway: indications that individual heterogeneity in risk is related to birth cohort. Eur J Epidemiol. 2006;21(8):587–93. doi: 10.1007/s10654-006-9043-8. [DOI] [PubMed] [Google Scholar]
- 39.Stanta G, Campagner L, Cavallieri F, Giarelli L. Cancer of the oldest old. What we have learned from autopsy studies. Clin Geriatr Med. 1997;13(1):55–68. [PubMed] [Google Scholar]